Why Use a Steroid Hormone Panel?
Steroid hormones are cholesterol-derived signaling molecules (e.g., glucocorticoids, mineralocorticoids, androgens, estrogens, progestogens) that regulate metabolism, reproduction, immunity, and stress responses. Because steroid levels are dynamic and strongly influenced by sample matrix and biology, many projects benefit from panel-based profiling rather than single-analyte measurement.
Clients typically use a steroid panel to:
- Capture pathway readouts by quantifying intermediates and endpoints in one workflow
- Assess hormonal balance and multi-hormone shifts consistent with feedback regulation
- Integrate with multi-omics (transcriptomics/proteomics/metabolomics) for mechanism studies
- Compare conditions across treatments, genotypes, time points, or stress paradigms
Steroid Hormone Panel Analysis
- Targeted LC-MS/MS and GC-MS/MS Quantification – Ultra-sensitive measurement of specific steroid hormones, ideal for validating biomarkers or confirming pathway intermediates.
- Comprehensive Multi-Hormone Panels – Simultaneous profiling of glucocorticoids, mineralocorticoids, androgens, estrogens, progestogens, and their key precursors in a single run.
- Custom Hormone Analysis Design – Tailored target lists and detection parameters to address unique project requirements, from developmental studies to stress physiology.
- Stable Isotope-Labeled Internal Standards – Ensuring accuracy by compensating for matrix effects across all sample types.
- Pathway Mapping and Comparative Analysis – Linking hormone quantification results to KEGG/HMDB pathways for functional interpretation and experimental comparisons.
List of Detected Steroid Hormones and Related Analytes
| Steroid Class |
Representative Hormones |
Associated Detectable Metabolites / Precursors |
| Glucocorticoids |
Cortisol, Cortisone |
11-Deoxycortisol, 21-Deoxycortisol, Tetrahydrocortisol (THF), Allotetrahydrocortisol (allo-THF), Tetrahydrocortisone (THE), 6β-Hydroxycortisol |
| Mineralocorticoids |
Aldosterone, Deoxycorticosterone |
18-Hydroxycorticosterone, 11-Deoxycorticosterone (DOC), Tetrahydrodeoxycorticosterone (THDOC) |
| Androgens |
Testosterone, Dihydrotestosterone (DHT), Androstenedione |
Androsterone, Etiocholanolone, 3α-Androstanediol, 3β-Androstanediol, Dehydroepiandrosterone (DHEA), DHEA-Sulfate (DHEA-S) |
| Estrogens |
Estradiol (E2), Estrone (E1), Estriol (E3) |
2-Hydroxyestrone, 4-Hydroxyestrone, 16α-Hydroxyestrone, 2-Methoxyestrone, 2-Methoxyestradiol |
| Progestogens |
Progesterone, 17α-Hydroxyprogesterone |
20α-Dihydroprogesterone, 5α-Dihydroprogesterone, Pregnanediol, Pregnanetriol, Allopregnanolone |
| Precursors |
Pregnenolone, 17α-Hydroxypregnenolone |
Isopregnanolone, 21-Hydroxypregnenolone |
| Plant Steroids (optional panel) |
Brassinolide, Castasterone |
6-Deoxocastasterone, Teasterone, Typhasterol |
| Conjugated Forms (optional panel) |
Sulfated or glucuronidated derivatives of the above classes |
Estrone sulfate, Testosterone glucuronide, Cortisol sulfate |
Why Choose Our Steroid Hormone Analysis Service: Key Advantages
- Ultra-Sensitive Quantification – Limits of detection down to 2–5 pg/mL for most steroid hormones, enabling accurate profiling of low-abundance analytes.
- High Analytical Precision – Multi-point calibration with R² ≥ 0.995; intra- and inter-batch CV ≤ 10% to ensure data reproducibility.
- Comprehensive Hormone Panel – Simultaneous detection of >20 steroid hormones across glucocorticoid, mineralocorticoid, androgen, estrogen, and progestogen pathways.
- Stable Isotope Dilution – Isotope-labeled internal standards for each target to correct matrix effects and guarantee absolute quantification.
- Integrated Bioinformatics Output – Includes hormone ratio calculations, pathway-level mapping (KEGG/HMDB), and comparative statistical analysis.
Steroid Hormone Assay Technical Details & Coverage
Our steroid hormone assays are performed on high-sensitivity LC-MS/MS and GC-MS/MS platforms to deliver the selectivity and sensitivity required for accurate hormone quantification. Compared with single-stage LC-MS or GC-MS, tandem MS/MS allows selective monitoring of precursor-to-product ion transitions, reducing matrix interference and enabling reliable quantification of structurally similar compounds at pg/mL levels.
Instrumentation:
- LC-MS/MS – Sciex QTRAP® 6500+, Thermo TSQ Altis, Waters Xevo TQ-S, coupled with UHPLC systems using reverse-phase C18 columns (2.1 × 100 mm, 1.7 μm).
- GC-MS/MS – Agilent 7890B GC with 7000D Triple Quadrupole for volatile or derivatized steroids.
Detection Mode: Multiple Reaction Monitoring (MRM) with polarity switching to maximize analyte coverage.
Example Transitions: Testosterone (m/z 289 → 97), Estradiol (m/z 271 → 145), Cortisol (m/z 363 → 121), Progesterone (m/z 315 → 97).
LOD: Typically 2–5 pg/mL for most analytes; Dynamic Range: 5–6 orders of magnitude.
By combining LC-MS/MS for thermolabile or polar hormones and GC-MS/MS for volatile derivatives, our method ensures full coverage of glucocorticoids, mineralocorticoids, androgens, estrogens, progestogens, and their key precursors and metabolites.
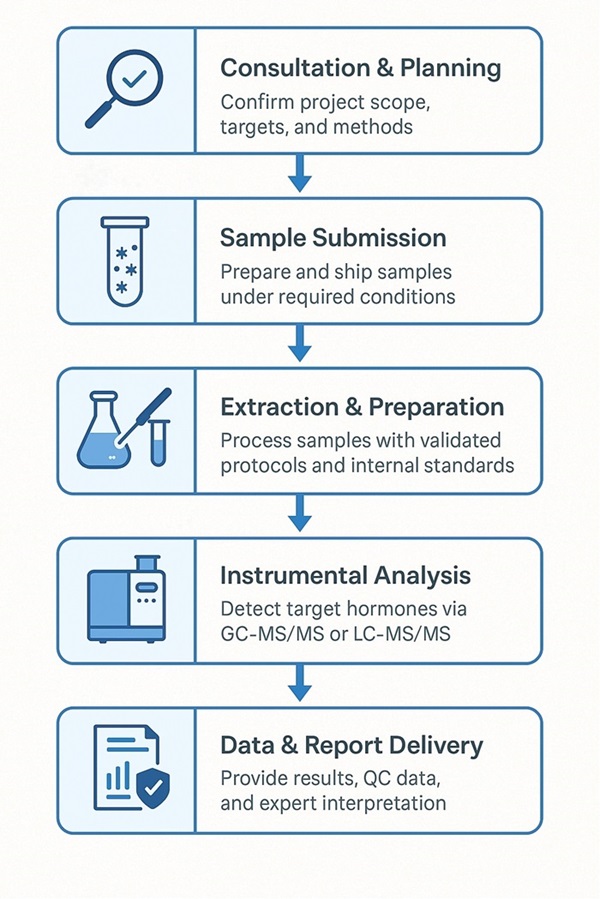
Step-by-Step Workflow for Steroid Hormone Analysis
How to Prepare Your Samples for Steroid Hormone Quantification
| Sample Type |
Recommended Amount/Volume |
Storage Condition |
Shipping Requirement |
Notes |
| Serum |
≥ 200 μL |
-80 °C |
Ship on dry ice |
Avoid repeated freeze–thaw cycles |
| Plasma |
≥ 200 μL |
-80 °C |
Ship on dry ice |
Specify anticoagulant type |
| Urine |
≥ 500 μL |
-80 °C |
Ship on dry ice |
Prefer first-morning urine; avoid bacterial contamination |
| Tissue |
≥ 50 mg |
-80 °C |
Ship on dry ice |
Pre-cool and grind into powder if possible |
| Cells |
≥ 1×10⁶ cells |
-80 °C |
Ship on dry ice |
Wash with PBS to remove culture medium |
| Cell Culture Supernatant |
≥ 500 μL |
-80 °C |
Ship on dry ice |
Filter to remove debris |
| Other Special Samples |
Project-specific assessment |
-80 °C |
Ship on dry ice |
Please contact our technical team in advance |
From Lab to Discovery: Applications of Steroid Hormone Testing
Deliverables: What You Get from Our Steroid Hormone Analysis Service
Raw Data Files
- Original GC-MS/MS or LC-MS/MS spectral files
- Instrument parameters and acquisition settings
Quantitative/Semi-Quantitative Results Table
- Concentrations of targeted steroid hormones (ng/mL or ng/g)
- Internal standard correction data and detection limits (LOD/LOQ)
Quality Control (QC) Report
- Signal intensity, peak shape, and retention time assessments
- Standard curves with correlation coefficients (R² values)
- Method repeatability and stability validation
Optional Statistical and Visualization Analysis
- Comparative analysis between sample groups (t-test, ANOVA, etc.)
- Multivariate analysis (PCA, PLS-DA)
- Visual outputs such as heatmaps and volcano plots
Comprehensive Project Report (PDF)
- Detailed description of experimental methods and parameters
- Summary of detection results
- Data interpretation and key conclusions
Estrogen–Microbiota Link Revealed by Steroid Hormone Profiling
Background
Sex differences in gut microbiota arise from both sex hormones and sex chromosomes. Disentangling their roles requires models that decouple gonadal sex from XX/XY complement.
Objective
Determine whether sex hormones or sex chromosomes primarily shape gut microbiota, and test whether Akkermansia muciniphila responds to estrogen.
Model & Methods
- Four-core genotype (FCG) mice (XX or XY with ovaries; XX or XY with testes), 15–16 weeks; n=6–8/group.
- Gonadectomy to deplete circulating sex steroids.
- Plasma steroid quantification: LC-MS/MS (ESI-MRM) multi-hormone panel (Creative Proteomics); key readouts included estradiol, progesterone, testosterone and precursors/metabolites.
- Microbiota profiling: 16S rRNA sequencing; α/β diversity analyses.
- In vitro assay: growth of A. muciniphila ± β-estradiol (2.2–10 μM).
Key Findings
- Hormone depletion confirmed: ovariectomy lowered estradiol and progesterone in females (P<0.001/0.03); castration markedly reduced testosterone in males (P<0.0001).
- Diversity shifts: female α-diversity increased after gonadectomy regardless of XX/XY; male β-diversity differed between XX and XY.
- A. muciniphila is estrogen-responsive: abundance decreased in gonadectomized females vs intact (P<0.0001). β-estradiol dose-dependently increased A. muciniphila growth in vitro (P<0.001).
- Conclusion: Sex hormones override sex chromosomes in shaping gut microbiota in this model; estrogen positively associates with Akkermansia.
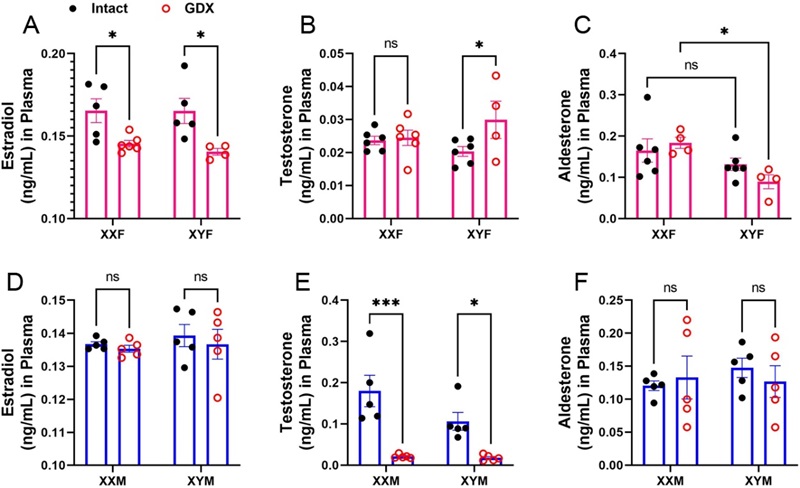 Plasma steroids after gonadectomy. Ovariectomy lowers female estradiol; aldosterone shows XX>XY effect. Castration reduces male testosterone; estradiol/aldosterone unchanged. Two-way ANOVA; n=4–6/group; *P<0.05.
Plasma steroids after gonadectomy. Ovariectomy lowers female estradiol; aldosterone shows XX>XY effect. Castration reduces male testosterone; estradiol/aldosterone unchanged. Two-way ANOVA; n=4–6/group; *P<0.05.
Impact for Researchers.
- Integrating steroid profiling (LC-MS/MS) with microbiome readouts can reveal hormone–microbe interactions that underlie metabolic and immune phenotypes.
- Supports study designs probing estrogen signaling, microbiota modulation, and pathway-level mechanisms in preclinical models.
Reference
- Sakamuri, Anil, et al. "Sex hormones, sex chromosomes, and microbiota: identification of Akkermansia muciniphila as an estrogen-responsive bacterium." Microbiota and host 1.1 (2023).
Inflammation primes the kidney for recovery by activating AZIN1 A-to-I editing
Heruye, S., Myslinski, J., Zeng, C., Zollman, A., Makino, S., Nanamatsu, A., ... & Hato, T.
Journal: bioRxiv
Year: 2023











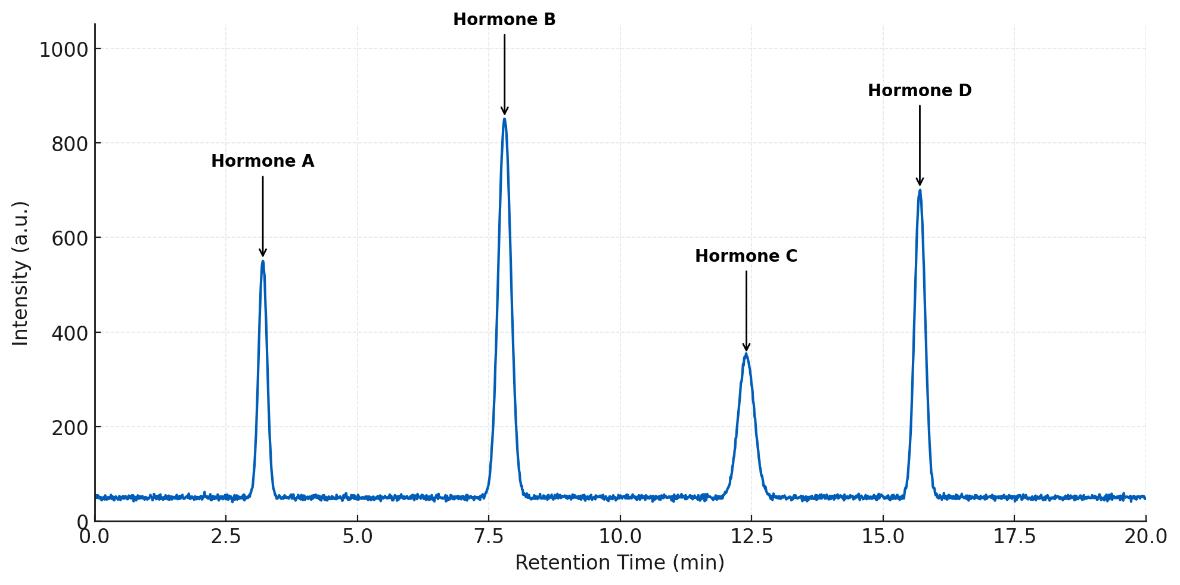
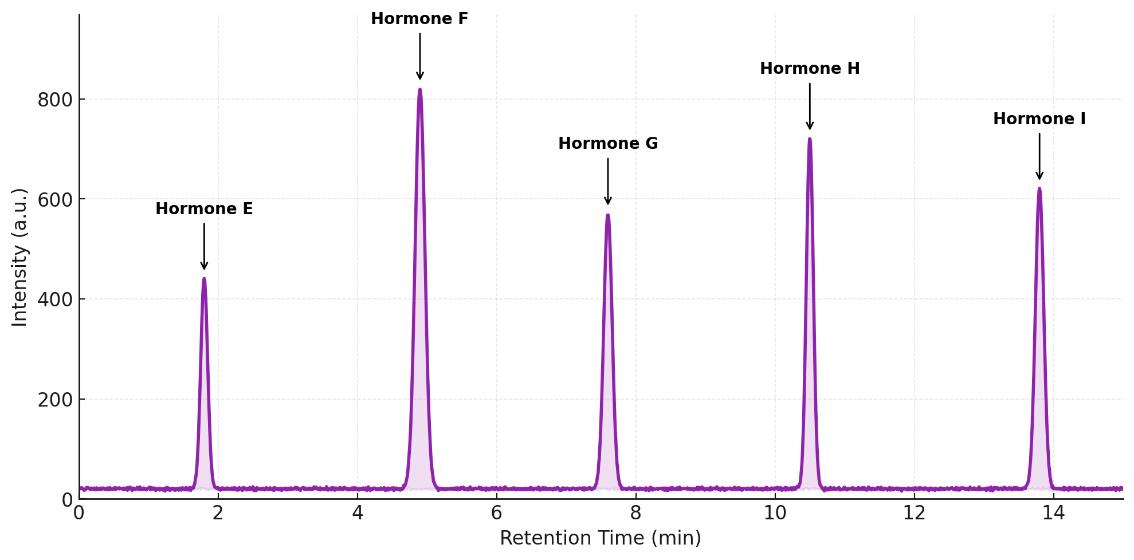
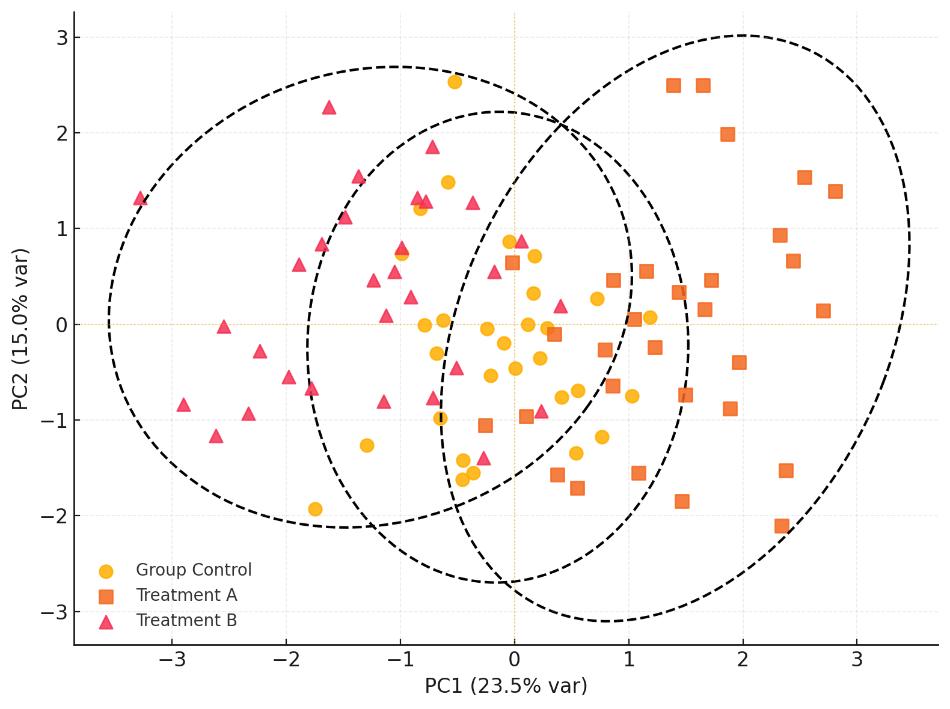
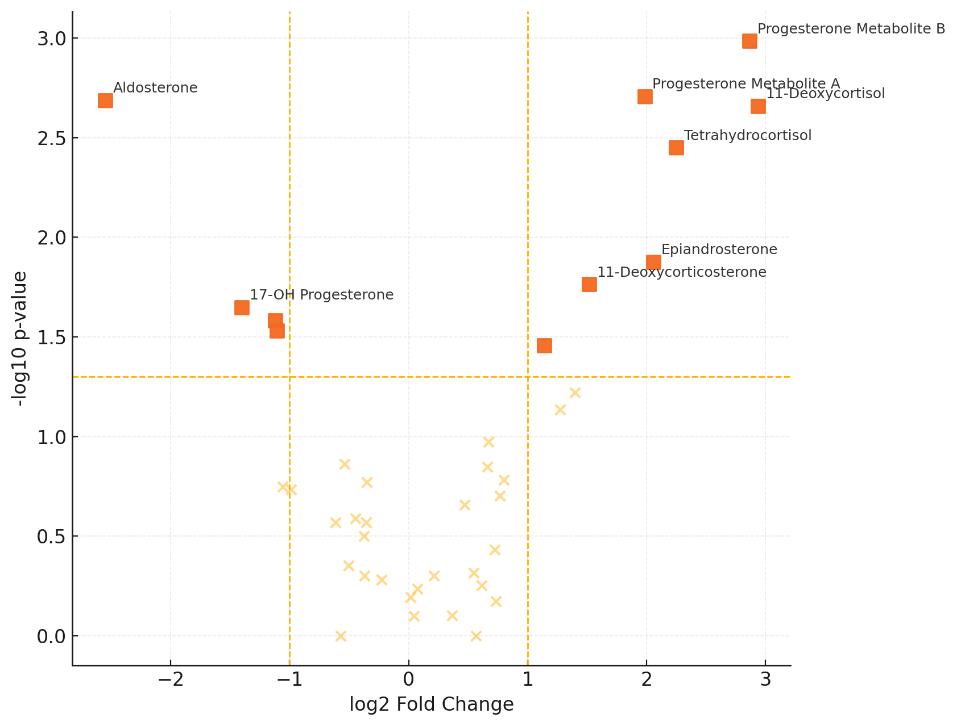
 Plasma steroids after gonadectomy. Ovariectomy lowers female estradiol; aldosterone shows XX>XY effect. Castration reduces male testosterone; estradiol/aldosterone unchanged. Two-way ANOVA; n=4–6/group; *P<0.05.
Plasma steroids after gonadectomy. Ovariectomy lowers female estradiol; aldosterone shows XX>XY effect. Castration reduces male testosterone; estradiol/aldosterone unchanged. Two-way ANOVA; n=4–6/group; *P<0.05.