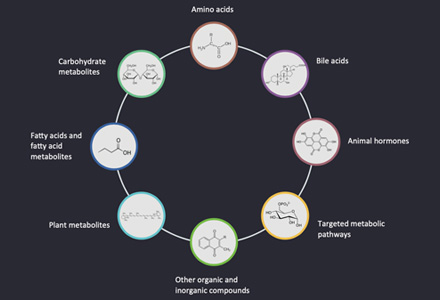
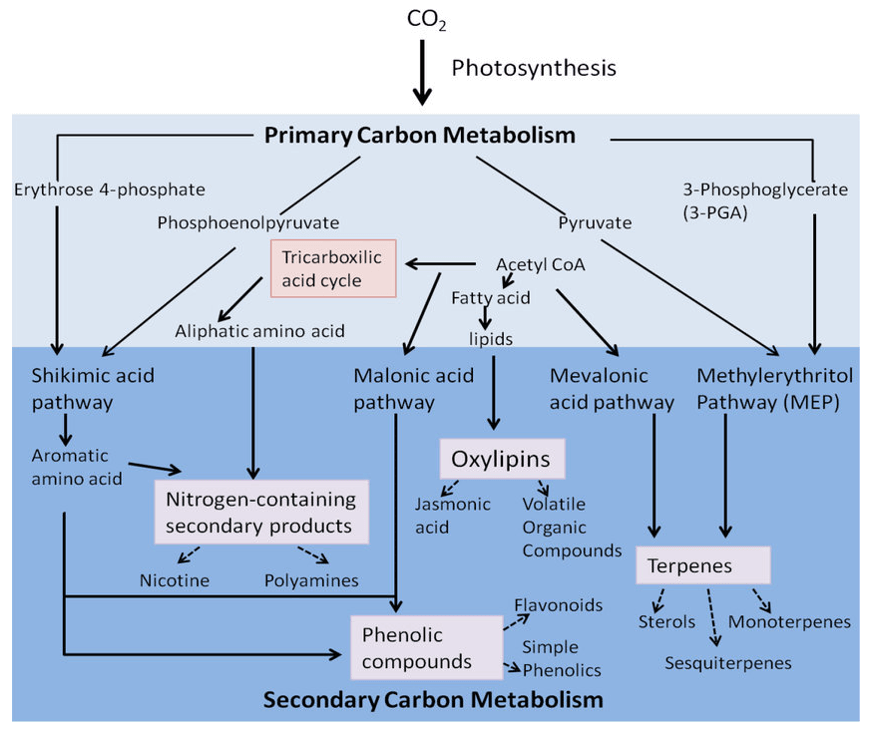
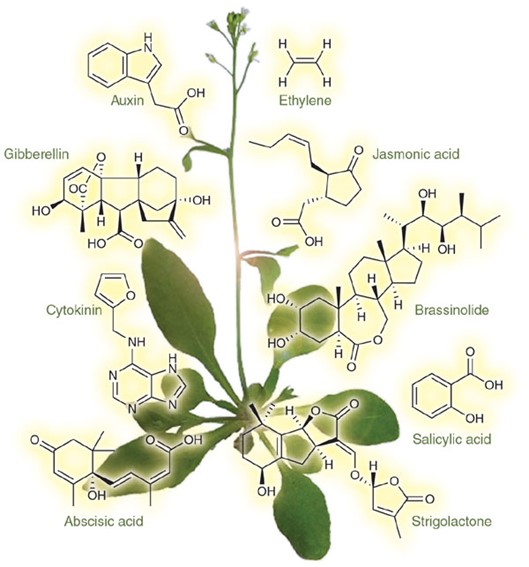
What is Plant Hormone?
Plant hormones are highly bioactive signaling molecules that act as chemical messengers, mediating and controlling physiological processes during plant growth and development as well as stress responses by additive, synergistic, or antagonistic actions. Plant hormones are often present in trace amounts and their levels depend strongly on the plant organ, plant developmental stage, and environmental conditions. Plant hormones are structurally diverse active compounds that are divided into several major groups including auxins, cytokinins (CTK), gibberellins (GA), abscisic acid (ABA), ethylene (ETH), jasmonate (JA), salicylic acid (SA), strigolactone (SL) and brassinosteroids (BR). Mass spectrometry is a sensitive analytical technique that enables detection, identification and quantification of a variety of plant hormones by measuring their mass and characterizing their chemical structure, even at low abundance. MS instruments combine with either gas chromatography (GC) or liquid chromatography (LC) to effectively separate organic molecules from complex samples. Comprehensive phytohormone profiling helps elucidate a plant's responses to different conditions such as mutations, stress, and hormone treatments.
Applications of Plant Hormones Analysis
Understanding Growth and Development: Analyzing hormone levels helps researchers understand plant growth and development mechanisms.
Environmental Responses: Hormonal signaling reveals how plants adapt to light, temperature, and stress.
Crop Improvement: Manipulating hormone levels enhances crop yield and quality.
Disease and Pest Resistance: Hormone analysis identifies targets for developing disease-resistant crops.
Senescence and Ripening: Ethylene analysis manages post-harvest life of fruits and vegetables.
Root Development and Nutrient Uptake: Studying hormone dynamics in roots informs nutrient uptake strategies.
Environmental Monitoring: Hormones indicate environmental stress on plants.
Biotechnological Applications: Essential for tissue culture and plant transformation.
Ecological Studies: Hormones contribute to plant-plant communication and ecological interactions.
Advantages of Our Plant Hormones Analysis Service
Rich experience in plant sample handling and analysis of various plant hormones
Enable analysis of plant hormones present in a variety of plant species at cell/tissue level
Accurate quantification of plant hormones with LC-MS and GC-MS
Flexible statistical analysis and bioinformatics analysis
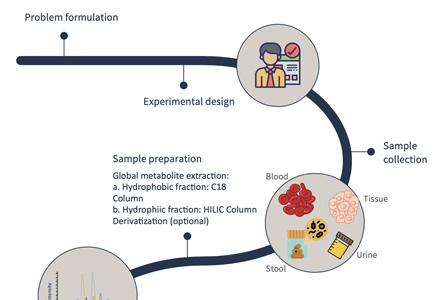
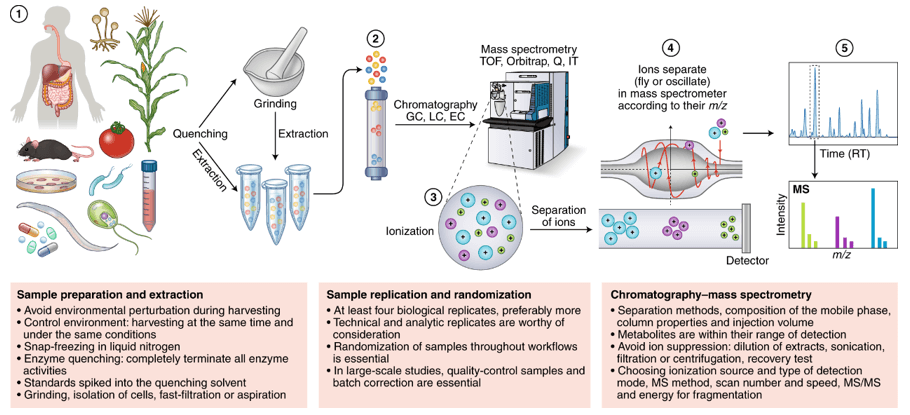
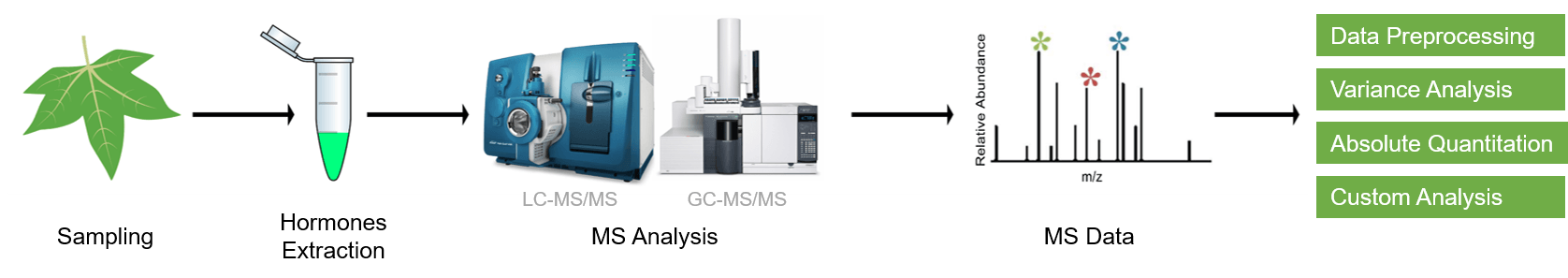
Workflow of Plant Hormones Analysis
Since different tissues have different matrix properties, Creative Proteomics has developed multiple novel sample preparation techniques. Our services allow the profiling of more than 100 plant hormones, including auxins, GAs, CKs, ABA, BRs, JAs, SA, etc.
Mode: MRM
Precision: pmol
Linear: R2 > 0.99
Analysis content:
- Standard curve creation
- Raw data preprocessing
- Absolute quantification of phytohormones
- Differential metabolites screening
- Optimal analyses such as KEGG pathway analysis and hierarchical clustering
List of Detectable Plant Hormones at Creative Proteomics
| Category | Specific Hormones |
|---|
| Auxins | Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA), 1-Naphthaleneacetic acid (NAA) |
| Abscisic acid | ABA |
| Brassinosteroids | Brassinolide, Castasterone |
| Cytokinins | Zeatin, Isopentenyladenine (IPA), 6-Benzylaminopurine (BAP), Dimethylallyladenine (DZ) |
| Ethylene | Ethylene |
| Gibberellins | GA1, GA3, GA4, GA5, GA6, GA7, GA8, GA9, GA13, GA14, GA15, GA19, GA20, GA24, GA29, GA44, GA51, GA53 |
| Jasmonates | Jasmonic acid (JA), Methyl jasmonate (MeJA) |
| Salicylic acid | Salicylic acid (SA) |
| Strigolactones | Strigolactone |
| Other known hormones | Plant peptide hormones, Polyamines, Nitric oxide, Karrikins, Triacontanol, Phytosulfokine (PSK), Melatonin |
Sample Requirements of Plant Hormones Assay
Sample Types:
- Plant Tissues: Choose tissues relevant to the plant hormones under investigation, such as leaves, roots, or stems. Ensure that fresh samples are collected as hormone levels may change over time.
- Cultures: If working with in vitro cultures of plant cells or tissues, collect samples from the culture medium containing the plant tissues.
Recommended Sample Quantities:
- Plant Tissues: The sample quantity depends on the plant species and tissue type. Generally, it is advisable to use approximately 0.1-0.5 grams of fresh tissue.
- Cultures: Sample quantity depends on the scale and type of the culture. Ensure that the sample size is sufficient for the chosen analytical methods.
Deliverables
- Experimental procedure
- Parameters of liquid chromatography and MS
- MS raw data files and MS data quality checks
- Metabolites quantification data
- Custom analysis report
Mass spectrometry-based profiling of plant hormones enables quantitative analyses of plant hormones in a faster, convenient, and sensitive manner. With decades of experience in mass spectrometry services, Creative Proteomics has a proven track record supporting diverse plant hormone detection and quantification. We can meet your specific project requirements, from sampling to bioinformatics.
References
- Ondřej Novák, Richard Napier, Karin Ljung. Zooming In on Plant Hormone Analysis: Tissue- and Cell-Specific Approaches. Annu Rev Plant Biol, 2017 Apr 28(68):323-348.
- Xiangqing Pan, Xuemin Wang. Profiling of plant hormones by mass spectrometry. Journal of Chromatography B Analytical Technologies in the Biomedical & Life ences, 2009, 877(26):2806-2813.
Case: Integrated Analysis of Transcriptome and Hormone Profiling Reveals Dynamic Responses to Moderate Dehydration Stress in Arabidopsis.
Background:
Plant responses to dehydration stress involve complex molecular and hormonal changes. A two-phased response, comprising early stomatal closure and late induction of protective mechanisms, is orchestrated by the hormone abscisic acid (ABA). This study aims to elucidate the temporal dynamics of gene expression and hormone profiles during moderate dehydration stress in Arabidopsis, with a focus on the role of ABA.
Samples:
Arabidopsis thaliana ecotype Col-0 (WT) and the ABA biosynthetic mutant nced3-2 were employed. Moderate dehydration stress was induced in 3-week-old plants, allowing the separation of early and late stress responses.
Technical Methods
Plant Growth and Stress Treatment:
- WT and nced3-2 plants grown in soil tablets under controlled conditions.
- Moderate dehydration stress imposed by withholding water for 3-72 hours.
Transcriptome Analysis:
- Agilent Arabidopsis 4 Oligo Microarray used for gene expression analysis.
- RNA extracted from plants at various time points during dehydration.
- Data analyzed using Feature Extraction and GeneSpring GX software.
Quantitative Real-Time PCR (qRT-PCR):
- Validation of gene expression via qRT-PCR.
- Primers designed based on AGI codes.
- Normalization with 18S rRNA and At2g32170.
Plant Hormone Profiling:
- Hormone extraction and purification from plant materials.
- LC-ESI-MS/MS apparatus 6410 used for hormone analysis.
- Internal standards (D6-ABA, D5-tZ, D2-IAA, D6-SA, D2-JA, D2-GA4) applied.
- Multiple fractions and columns utilized for comprehensive hormone profiling.
Data Deposition:
- Microarray design and data deposited at ArrayExpress (accession number E-MTAB-4640).
Results
- Bi-phasic ABA accumulation observed during moderate dehydration stress.
- Dynamic changes in the transcriptome, with distinct early and late response phases.
- Coordinated regulation of ABA biosynthetic and responsive genes.
- Close relationship between ABA and jasmonic acid (JA) signaling pathways.
- Identification of touch stress-responsive genes and involvement of AP2/ERF transcription factors in early dehydration response.
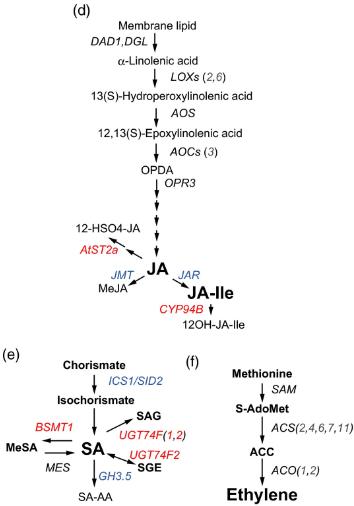 (d) Biosynthetic pathway of (d) JA, (e) SA and (f) ET in Arabidopsis.
(d) Biosynthetic pathway of (d) JA, (e) SA and (f) ET in Arabidopsis.
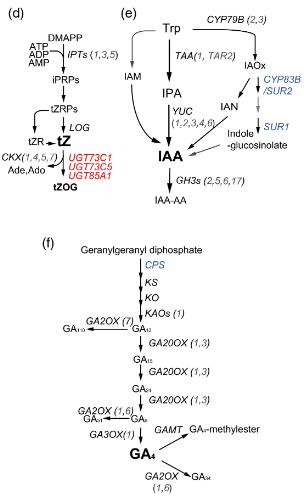 (d–f) Biosynthetic pathway of (d) tZ, (e) IAA, and (f) GA4 in Arabidopsis
(d–f) Biosynthetic pathway of (d) tZ, (e) IAA, and (f) GA4 in Arabidopsis
Reference
- Urano, Kaoru, et al. "Analysis of plant hormone profiles in response to moderate dehydration stress." The Plant Journal 90.1 (2017): 17-36.


 (d) Biosynthetic pathway of (d) JA, (e) SA and (f) ET in Arabidopsis.
(d) Biosynthetic pathway of (d) JA, (e) SA and (f) ET in Arabidopsis. (d–f) Biosynthetic pathway of (d) tZ, (e) IAA, and (f) GA4 in Arabidopsis
(d–f) Biosynthetic pathway of (d) tZ, (e) IAA, and (f) GA4 in Arabidopsis