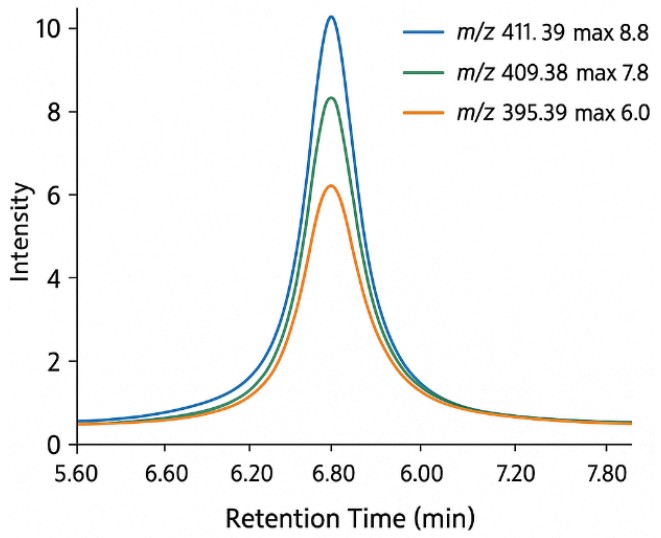
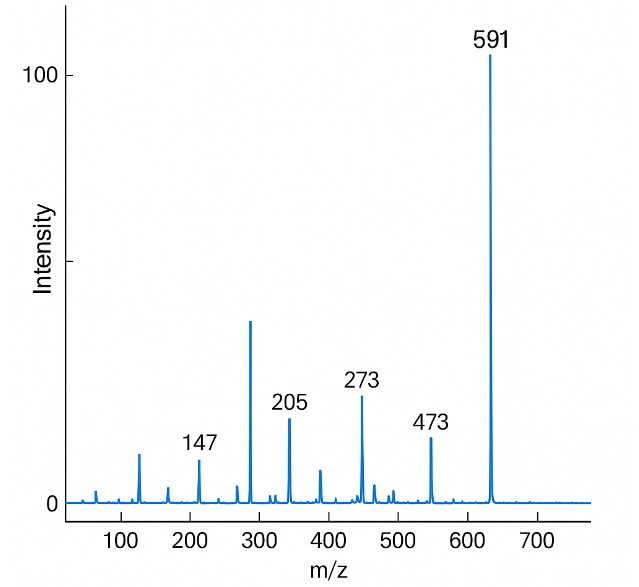
What Is the Mevalonate Pathway and Why Analyze It
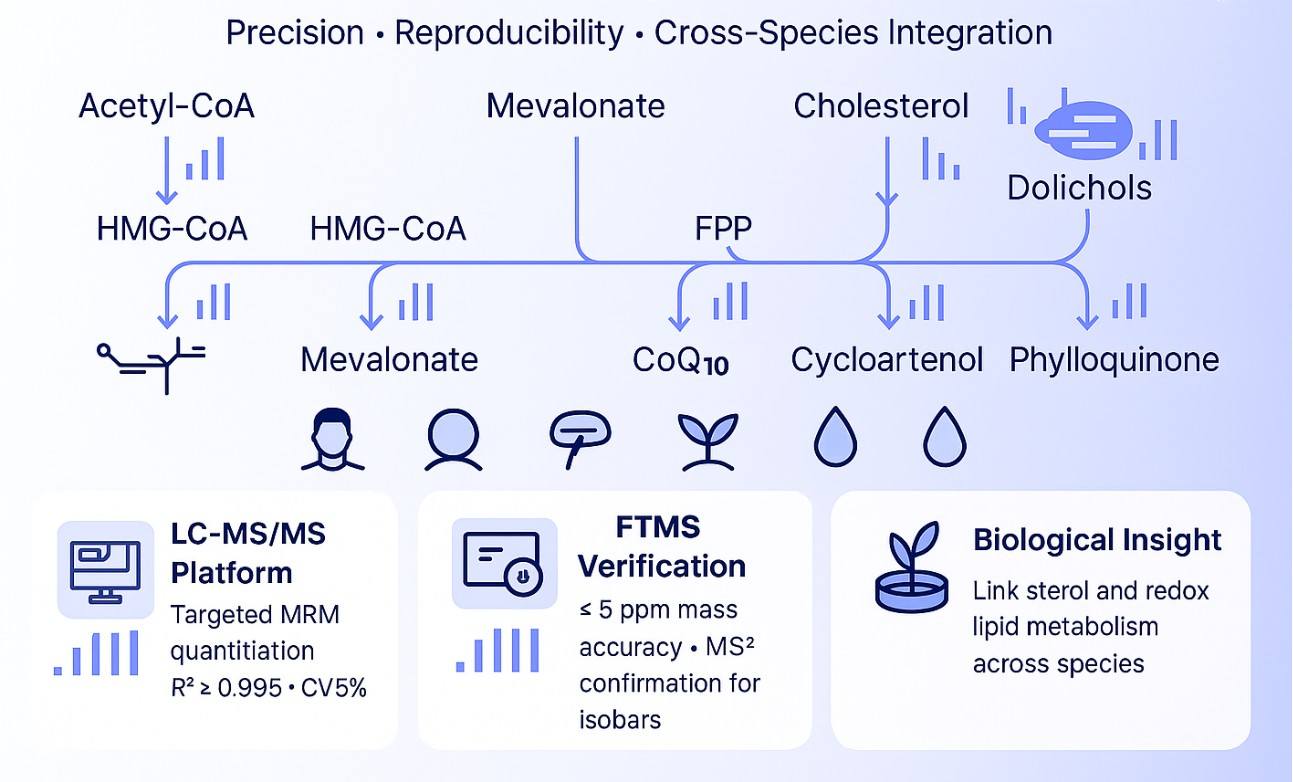
The mevalonate (MVA) pathway is a central metabolic route that supplies precursors for isoprenoid biosynthesis in mammals, plants, and microorganisms. Together with the plastidial MEP/DOXP pathway in plants, it generates isoprenyl diphosphates that support essential cellular processes such as protein prenylation, membrane assembly, and stress adaptation.
These pathways give rise to biologically important lipid classes, including sterols (cholesterol in mammals, phytosterols in plants), ubiquinones (CoQ), plastoquinone, dolichols or polyprenols, and vitamin K compounds. Measuring these intermediates provides a direct readout of pathway activity.
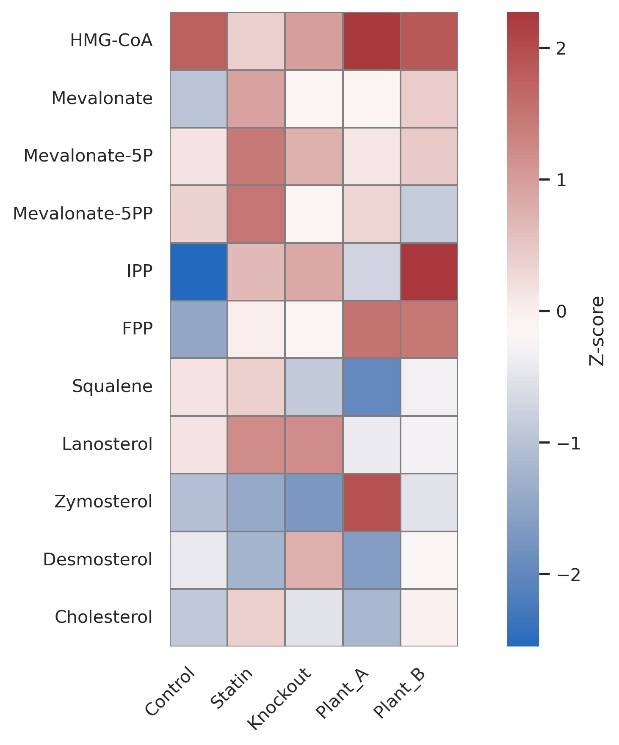
Quantitative analysis of the mevalonate network helps reveal metabolic bottlenecks, lipid synthesis capacity, and responses to genetic or compound intervention. It is widely used to study mitochondrial function, redox balance, membrane biology, and growth regulation. Targeted LC–MS profiling enables precise measurement of pathway intermediates across diverse sample types for unbiased biological interpretation.
What Problems We Help You Solve
- Dissect regulatory bottlenecks across MVA and MEP networks
Quantifying key intermediates helps reveal control points in sterol and isoprenoid metabolism.
- Connect pathway activity to genetic or compound perturbations
Use defined metabolite shifts to validate enzyme targeting or expression-driven metabolic rewiring.
- Resolve structurally related metabolites in complex matrices
Confidently distinguish closely eluting or isobaric species across sterol, quinone, and prenol lipid classes.
- Integrate plant and mammalian samples into a unified analytical framework
Matrix-matched methods allow you to compare across species, tissues, and experimental systems.
- Support studies in redox function, growth regulation, and membrane biology
Quantitative profiles of pathway products link upstream biosynthesis to functional outputs in mitochondria, plastids, and ER.
How Mevalonate Pathway Analysis Supports Your Research
Reveal control points in isoprenoid metabolism
Quantify HMG-CoA, mevalonate derivatives, and prenyl diphosphates to locate pathway bottlenecks under compound treatment, nutrient shifts, or genetic manipulation.
Evaluate sterol biosynthesis in animal and plant systems
Track sterol intermediates such as lanosterol, zymosterol, desmosterol, sitosterol, and stigmasterol to assess flux, accumulation, or desaturation activity.
Understand redox-linked lipid pathways
Analyze CoQ (CoQ6–CoQ12), plastoquinone, and phylloquinone chain-length variants to explore mitochondrial and chloroplast redox potential.
Estimate prenylation and membrane-targeting potential
Measure farnesyl and geranylgeranyl diphosphates to gauge prenylation capacity and its role in signaling or membrane association.
Connect pathway data to broader lipidomics
Integrate targeted MVA/MEP outputs with untargeted lipidomics or redox panels to build system-level insight into lipid biosynthesis and organelle function.
Detectable Mevalonate Pathway Analyte List (Complete Panel)
Our panel includes key intermediates, diphosphates, sterol precursors, and lipid end-products from both the mevalonate (MVA) and MEP/DOXP pathways. Coverage spans mammalian and plant-specific metabolites.
Core Mevalonate Pathway Intermediates
| Compound |
Superclass |
Class |
| Acetyl-CoA |
Lipids and lipid-like molecules |
Fatty acyls |
| Acetoacetyl-CoA |
Lipids and lipid-like molecules |
Fatty acyls |
| 3-Hydroxy-3-methylglutaryl-CoA (HMG-CoA) |
— |
— |
| Mevalonic acid |
Lipids and lipid-like molecules |
Fatty acyls |
| Mevalonic acid 5-phosphate |
— |
— |
| Mevalonic acid 5-pyrophosphate |
Organic oxygen compounds |
Organic oxoanionic compounds |
MEP Pathway Intermediates (Plant Only)
| Compound |
Superclass |
Class |
| 1-Deoxy-D-xylulose 5-phosphate (DXP) |
Organic oxygen compounds |
Sugar phosphates |
| Methylerythritol phosphate (MEP) |
— |
— |
| CDP-ME |
— |
— |
| CDP-ME2P |
— |
— |
| MEcPP |
— |
— |
| HMBPP |
— |
— |
Prenyl Diphosphates (IPP, DMAPP, GPP, FPP, GGPP)
| Compound |
Superclass |
Class |
| Isopentenyl pyrophosphate (IPP) |
— |
— |
| Dimethylallyl pyrophosphate (DMAPP) |
Lipids and lipid-like molecules |
Prenol lipids |
| Geranyl pyrophosphate (GPP) |
Lipids and lipid-like molecules |
Prenol lipids |
| Farnesyl pyrophosphate (FPP) |
Lipids and lipid-like molecules |
Prenol lipids |
| Geranylgeranyl pyrophosphate (GGPP) |
Lipids and lipid-like molecules |
Prenol lipids |
Sterol Precursors and Cholesterol
| Compound |
Superclass |
Class |
Source |
| Lanosterol |
Lipids and lipid-like molecules |
Prenol lipids |
Mammal (MVA) |
| Dihydrolanosterol |
Lipids and lipid-like molecules |
Prenol lipids |
Mammal |
| Zymosterol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| Zymostenol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| Lathostenol |
— |
— |
Mammal |
| 7-Dehydrodesmosterol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| 7-Dehydrocholesterol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| Desmosterol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| Cholesterol |
Lipids and lipid-like molecules |
Steroids and steroid derivatives |
Mammal |
| Cycloartenol |
Lipids and lipid-like molecules |
Steroids |
Plant (MVA) |
| 24-Methylene cycloartanol |
— |
— |
Plant |
| Campesterol |
— |
Steroids and steroid derivatives |
Plant |
| Sitosterol |
— |
Steroids and steroid derivatives |
Plant |
| Stigmasterol |
— |
Steroids and steroid derivatives |
Plant |
Squalene and Oxidosqualene
| Compound |
Superclass |
Class |
| Squalene |
Lipids and lipid-like molecules |
Prenol lipids |
| 2,3-Oxidosqualene |
Lipids and lipid-like molecules |
Prenol lipids |
Coenzyme Q, Plastoquinones, Dolichols, and Polyprenols
| Compound |
Superclass |
Class |
Source |
| CoQ6–CoQ12 |
— |
— |
Both |
| Dolichols (13–21 isoprene units) |
— |
— |
Mammal |
| Polyprenols (varied chain length) |
— |
— |
Plant |
| Plastoquinone (PQ-9, PQ-10) |
— |
— |
Plant |
| Phylloquinone (Vitamin K1) |
— |
— |
Plant |
| Menaquinones (Vitamin K2 variants) |
— |
— |
Mammal |
Why Choose Our Mevalonate Pathway Analysis Service?
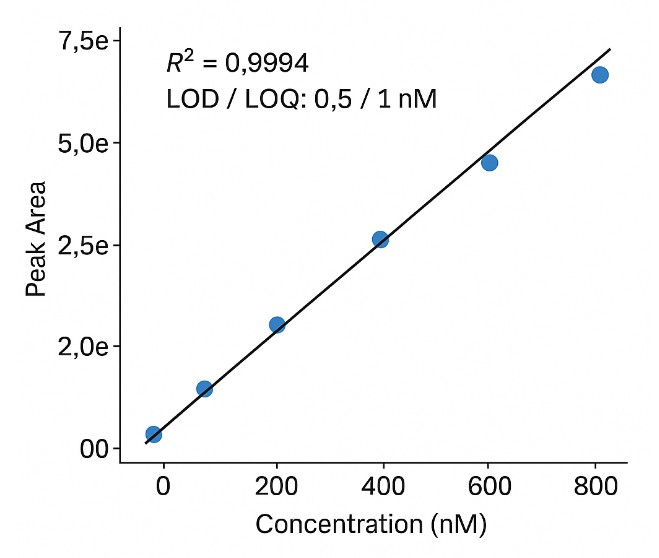
Standard curves with R2 ≥ 0.995 ensure reliable linearity across concentration ranges.
Intra- and inter-batch precision maintained at CV ≤ 15% for qualified targets.
Stabilized methods support low-abundance intermediates like phosphorylated species and dolichols.
- High-Confidence Identification
Optional FTMS offers ≤ 5 ppm mass accuracy and MS2 validation for structural confirmation.
- Low Background and Carryover
Signal carryover controlled under 1%; blanks confirm system cleanliness.
- Multi-Matrix Compatibility
Protocols adapted for plasma, cells, tissues, feces, and DBS to support diverse study designs.
Which Methods Are Used in Mevalonate Pathway Profiling?
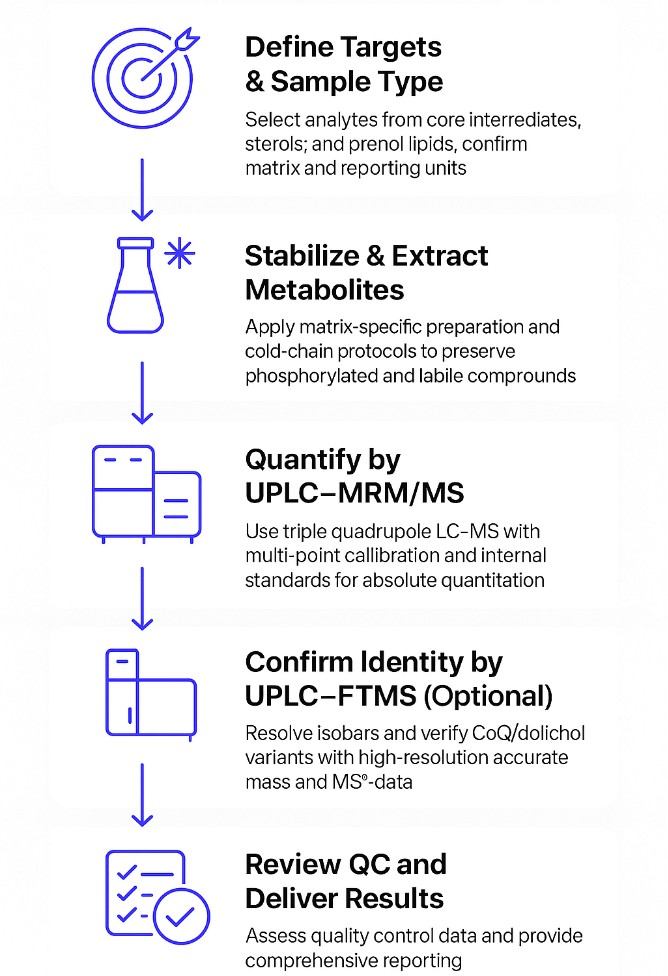
We apply advanced LC–MS instrumentation and method-specific optimizations to ensure accurate, reproducible quantification of mevalonate pathway metabolites. All analyses are performed under stringent quality control parameters.
UPLC–MRM/MS for Absolute Quantitation
Platform: Agilent 6495C quadrupole mass spectrometer
Mode: Scheduled MRM, polarity switching
Calibration: Multi-point external curve (R2 ≥ 0.995), with isotope-labeled or class-matched internal standards
Typical CVs: ≤ 15% for qualified targets
Use: Core quantitation of HMG-CoA, mevalonate derivatives, isoprenyl pyrophosphates, and sterols; includes support for MEP-derived intermediates in plant samples
UPLC–FTMS for Identity Confirmation (Optional)
Platform: Thermo Orbitrap series (e.g., Q Exactive, Exploris)
Mode: Full-scan HRAM with PRM or data-dependent MS2
Resolution: ≥ 60,000 at m/z 200; mass error ≤ 5 ppm
Mass accuracy: ≤ 5 ppm
Use: Structural verification of isobars (e.g., squalene vs. oxidosqualene), and resolution of CoQ, dolichol, plastoquinone, and phytosterol homologs
Chromatography Conditions
Columns: C18 or HILIC (2.1 × 100 mm, sub-2 μm particle size)
Mobile Phases: Aqueous ammonium formate or acetate (pH-controlled); acetonitrile or methanol as organic modifier
Flow Rate & Gradient: Optimized for analyte class; supports ≥10 points per peak in MRM
Matrix Compatibility: Suitable for polar (phosphates) and nonpolar (sterols, quinones) fractions from both mammalian and plant tissues
Mevalonate Pathway Analysis Workflow: Step by Step
Sample Requirements for Mevalonate Pathway Profiling
| Sample Type |
Min. Amount Required |
Notes |
| Animal tissues |
≥ 50 mg wet weight |
Snap-frozen preferred. Avoid freeze-thaw. |
| Cultured cells |
≥ 1×106 cells (pellet) |
Wash with PBS and snap-freeze. |
| Plasma/serum |
≥ 100 μL |
EDTA or heparinized. Avoid hemolysis. |
| Feces (mouse/human) |
≥ 50 mg |
Freeze immediately after collection. |
| Dried blood spots (DBS) |
3–5 punches (3 mm) |
Use Whatman 903 or equivalent. Store dry with desiccant. |
| Microbial pellets |
≥ 108 cells |
Harvest during mid-log phase for optimal metabolite content. |
| Plant tissues |
≥ 100 mg fresh/frozen |
Flash-freeze in liquid nitrogen. Avoid enzymatic degradation. |
| Plant callus or cell cultures |
≥ 50 mg |
Rapid harvest and freeze. Submit culture conditions if possible. |
| Lysates or extracts |
≥ 50 μL or ≥ 50 μg protein |
Buffer composition must be disclosed; avoid detergents or chelators. |
Shipping Tips:
All biological samples should be shipped on dry ice unless pre-dried (e.g., DBS). Label clearly, include sample manifest, and use leak-proof containers. For global shipments, ensure customs documentation matches declared contents.
What You Receive: Deliverables from Mevalonate Pathway Analysis
- Absolute metabolite concentrations (nmol/mg or nmol/mL) in Excel/CSV
- Annotated chromatograms with retention time and transitions (PDF)
- Calibration curves and QC summary (R2, CV%, LOD/LOQ)
- Method summary with instrumentation and workflow details
- Optional MS/MS spectra for identity confirmation
- Optional interpretive report linking results to pathway insights
Applications of Mevalonate Pathway Analysis in Research and Industry
MS-CETSA functional proteomics uncovers new DNA-repair programs leading to Gemcitabine resistance
Nordlund, P., Liang, Y. Y., Khalid, K., Van Le, H., Teo, H. M., Raitelaitis, M., ... & Prabhu, N.
Journal: Research Square
Year: 2024
DOI: https://doi.org/10.21203/rs.3.rs-4820265/v1
High Levels of Oxidative Stress Early after HSCT Are Associated with Later Adverse Outcomes
Cook, E., Langenberg, L., Luebbering, N., Ibrahimova, A., Sabulski, A., Lake, K. E., ... & Davies, S. M.
Journal:Transplantation and Cellular Therapy
Year: 2024
DOI: https://doi.org/10.1016/j.jtct.2023.12.096
Multiomics of a rice population identifies genes and genomic regions that bestow low glycemic index and high protein content
Badoni, S., Pasion-Uy, E. A., Kor, S., Kim, S. R., Tiozon Jr, R. N., Misra, G., ... & Sreenivasulu, N.
Journal: Proceedings of the National Academy of Sciences
Year: 2024
DOI: https://doi.org/10.1073/pnas.2410598121
The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner
Norman, J. E., Milenkovic, D., Nuthikattu, S., & Villablanca, A. C.
Journal: International Journal of Molecular Sciences
Year: 2024
DOI: https://doi.org/10.3390/ijms25063475
UDP-Glucose/P2Y14 Receptor Signaling Exacerbates Neuronal Apoptosis After Subarachnoid Hemorrhage in Rats
Kanamaru, H., Zhu, S., Dong, S., Takemoto, Y., Huang, L., Sherchan, P., ... & Zhang, J. H.
Journal: Stroke
Year: 2024
DOI: https://doi.org/10.1161/STROKEAHA.123.044422
Pan-lysyl oxidase inhibition disrupts fibroinflammatory tumor stroma, rendering cholangiocarcinoma susceptible to chemotherapy
Burchard, P. R., Ruffolo, L. I., Ullman, N. A., Dale, B. S., Dave, Y. A., Hilty, B. K., ... & Hernandez-Alejandro, R.
Journal: Hepatology Communications
Year: 2024
DOI: https://doi.org/10.1097/HC9.0000000000000502
Comparative metabolite profiling of salt sensitive Oryza sativa and the halophytic wild rice Oryza coarctata under salt stress
Tamanna, N., Mojumder, A., Azim, T., Iqbal, M. I., Alam, M. N. U., Rahman, A., & Seraj, Z. I.
Journal: Plant‐Environment Interactions
Year: 2024
DOI: https://doi.org/10.1002/pei3.10155
Teriflunomide/leflunomide synergize with chemotherapeutics by decreasing mitochondrial fragmentation via DRP1 in SCLC
Mirzapoiazova, T., Tseng, L., Mambetsariev, B., Li, H., Lou, C. H., Pozhitkov, A., ... & Salgia, R.
Journal: iScience
Year: 2024
DOI: https://doi.org/10.1016/j.isci.2024.110132
Physiological, transcriptomic and metabolomic insights of three extremophyte woody species living in the multi-stress environment of the Atacama Desert
Gajardo, H. A., Morales, M., Larama, G., Luengo-Escobar, A., López, D., Machado, M., ... & Bravo, L. A.
Journal: Planta
Year: 2024
DOI: https://doi.org/10.1007/s00425-024-04484-1
A personalized probabilistic approach to ovarian cancer diagnostics
Ban, D., Housley, S. N., Matyunina, L. V., McDonald, L. D., Bae-Jump, V. L., Benigno, B. B., ... & McDonald, J. F.
Journal: Gynecologic Oncology
Year: 2024
DOI: https://doi.org/10.1016/j.ygyno.2023.12.030
Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice
Chargo, N. J., Neugebauer, K., Guzior, D. V., Quinn, R. A., Parameswaran, N., & McCabe, L. R.
Journal: Frontiers in Cell and Developmental Biology
Year: 2024
DOI: https://doi.org/10.3389/fcell.2023.1324649
Proteolytic activation of fatty acid synthase signals pan-stress resolution
Wei, H., Weaver, Y. M., Yang, C., Zhang, Y., Hu, G., Karner, C. M., ... & Weaver, B. P.
Journal: Nature Metabolism
Year: 2024
DOI: https://doi.org/10.1038/s42255-023-00939-z
Quantifying forms and functions of intestinal bile acid pools in mice
Sudo, K., Delmas-Eliason, A., Soucy, S., Barrack, K. E., Liu, J., Balasubramanian, A., … & Sundrud, M. S.
Journal: bioRxiv
Year: 2024
DOI: https://doi.org/10.1101/2024.02.16.580658
Elevated SLC7A2 expression is associated with an abnormal neuroinflammatory response and nitrosative stress in Huntington's disease
Gaudet, I. D., Xu, H., Gordon, E., Cannestro, G. A., Lu, M. L., & Wei, J.
Journal: Journal of Neuroinflammation
Year: 2024
DOI: https://doi.org/10.1186/s12974-024-03038-2
Thermotolerance capabilities, blood metabolomics, and mammary gland hemodynamics and transcriptomic profiles of slick-haired Holstein cattle during mid lactation in Puerto Rico
Contreras-Correa, Z. E., Sánchez-Rodríguez, H. L., Arick II, M. A., Muñiz-Colón, G., & Lemley, C. O.
Journal: Journal of Dairy Science
Year: 2024
DOI: https://doi.org/10.3168/jds.2023-23878
Glycine supplementation can partially restore oxidative stress-associated glutathione deficiency in ageing cats
Ruparell, A., Alexander, J. E., Eyre, R., Carvell-Miller, L., Leung, Y. B., Evans, S. J., ... & Watson, P.
Journal: British Journal of Nutrition
Year: 2024
DOI: https://doi.org/10.1017/S0007114524000370
Untargeted metabolomics reveal sex-specific and non-specific redox-modulating metabolites in kidneys following binge drinking
Rafferty, D., de Carvalho, L. M., Sutter, M., Heneghan, K., Nelson, V., Leitner, M., ... & Puthanveetil, P.
Journal: Redox Experimental Medicine
Year: 2023
DOI: https://doi.org/10.1530/REM-23-0005
Sex modifies the impact of type 2 diabetes mellitus on the murine whole brain metabolome
Norman, J. E., Nuthikattu, S., Milenkovic, D., & Villablanca, A. C.
Journal: Metabolites
Year: 2023
DOI: https://doi.org/10.3390/metabo13091012
A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B
Liu, J. T., Doueiry, C., Jiang, Y. L., Blaszkiewicz, J., Lamprecht, M. P., Heslop, J. A., ... & Duncan, S. A.
Journal: Communications Biology
Year: 2023
DOI: https://doi.org/10.1038/s42003-023-04739-9
Methyl donor supplementation reduces phospho‐Tau, Fyn and demethylated protein phosphatase 2A levels and mitigates learning and motor deficits in a mouse model of tauopathy
van Hummel, A., Taleski, G., Sontag, J. M., Feiten, A. F., Ke, Y. D., Ittner, L. M., & Sontag, E.
Journal: Neuropathology and Applied Neurobiology
Year: 2023
DOI: https://doi.org/10.1111/nan.12931
Sex hormones, sex chromosomes, and microbiota: identification of Akkermansia muciniphila as an estrogen-responsive bacterium
Sakamuri, A., Bardhan, P., Tummala, R., Mauvais-Jarvis, F., Yang, T., Joe, B., & Ogola, B. O.
Journal: Microbiota and Host
Year: 2023
DOI: https://doi.org/10.1530/MAH-23-0010
Living in extreme environments: a photosynthetic and desiccation stress tolerance trade-off story, but not for everyone
Gajardo, H. A., Morales, M., López, D., Luengo-Escobar, M., Machado, A., Nunes-Nesi, A., ... & Bravo, L.
Journal: Authorea Preprints
Year: 2023
DOI: https://doi.org/10.22541/au.168311184.42382633/v2
Resting natural killer cell homeostasis relies on tryptophan/NAD+ metabolism and HIF‐1α
Pelletier, A., Nelius, E., Fan, Z., Khatchatourova, E., Alvarado‐Diaz, A., He, J., ... & Stockmann, C.
Journal: EMBO Reports
Year: 2023
DOI: https://doi.org/10.15252/embr.202256156
Function and regulation of a steroidogenic CYP450 enzyme in the mitochondrion of Toxoplasma gondii
Asady, B., Sampels, V., Romano, J. D., Levitskaya, J., Lige, B., Khare, P., ... & Coppens, I.
Journal: PLoS Pathogens
Year: 2023
DOI: https://doi.org/10.1371/journal.ppat.1011566