Acetyl-CoA Analysis Service
Submit Your InquiryWhat is Acetyl-CoA?
Acetyl-CoA is a central metabolite that plays a critical role in energy metabolism, biosynthesis, and epigenetic regulation. As a key intermediate in the tricarboxylic acid (TCA) cycle and a precursor for fatty acid and cholesterol synthesis, acetyl-CoA has a broad impact on cellular physiology and pathology. Therefore, the accurate quantification of acetyl-CoA levels and its metabolic fluxes is essential for understanding cellular metabolism and its dysregulation in diseases.
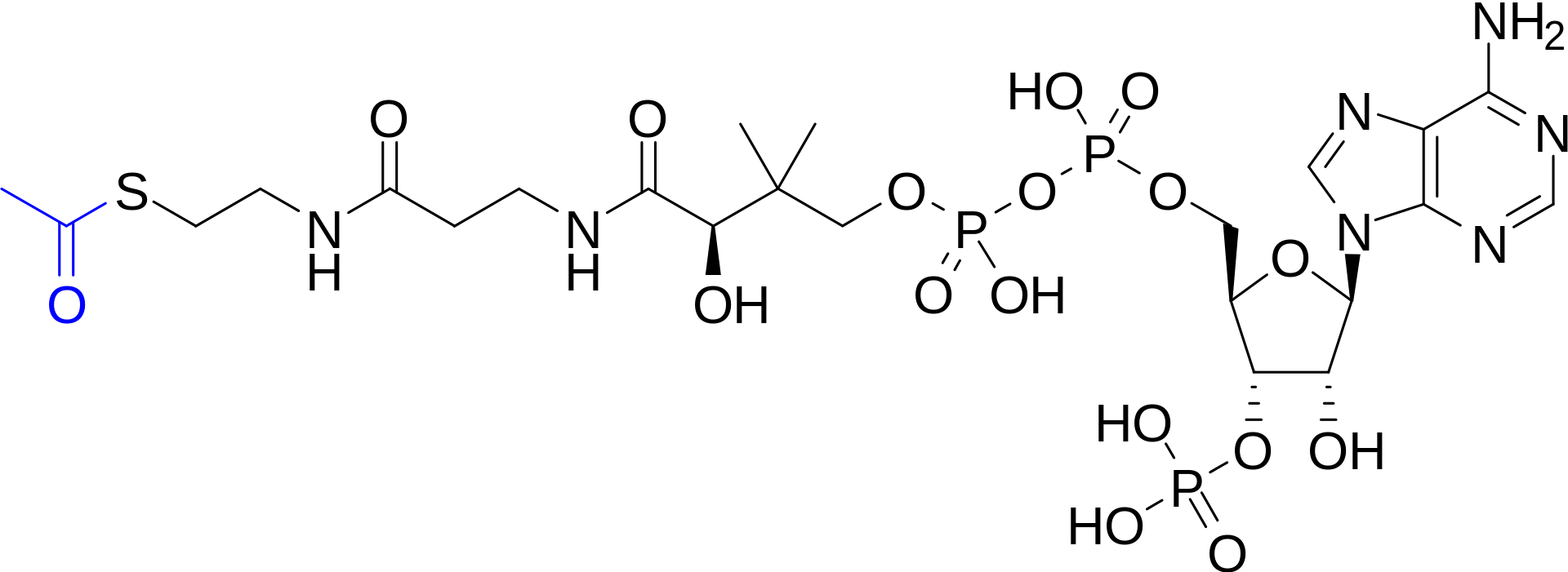 Molecular structure of acetyl-CoA
Molecular structure of acetyl-CoA
How can Creative Proteomics Support Your Acetyl-CoA Analysis?
At Creative Proteomics, we offer a range of analytical services to support your acetyl-CoA analysis needs, including:
Acetyl-CoA quantification: We use state-of-the-art mass spectrometry-based methods to accurately quantify acetyl-CoA levels and flux in biological samples, including cells, tissues, and bodily fluids.
Metabolic flux analysis: We can perform metabolic flux analysis to determine the rate of acetyl-CoA production and consumption in different metabolic pathways and under different conditions.
Targeted metabolomics: We offer targeted metabolomics services to analyze the levels of other metabolites in the same pathway as acetyl-CoA, providing a comprehensive picture of metabolic regulation and dysregulation.
Data analysis and interpretation: We provide advanced data analysis and interpretation services to help you make sense of your acetyl-CoA analysis results and identify potential targets for further study or therapeutic intervention.
Technical Route of Targeted Metabolomics of Acetyl-CoA
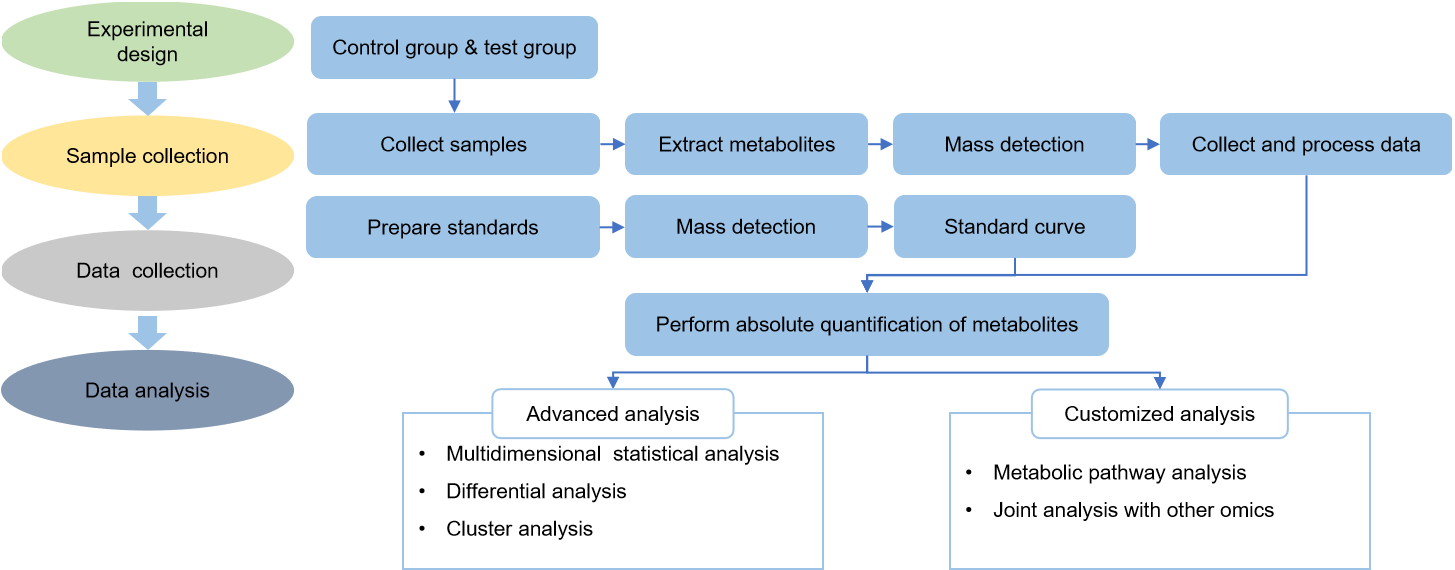
Technology Platform for Acetyl-CoA Analysis in Creative Proteomics
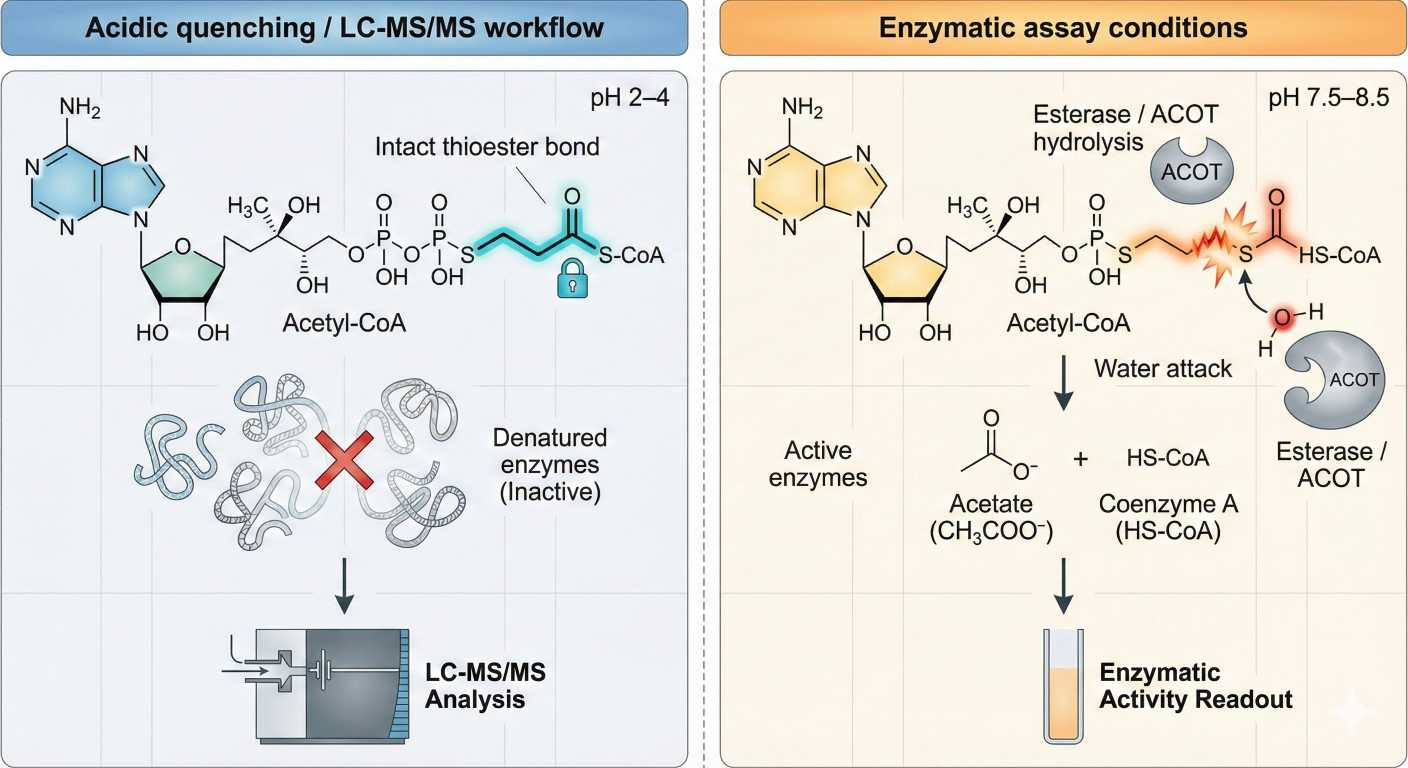
Liquid chromatography-mass spectrometry (LC-MS) is a powerful analytical technique that allows for the simultaneous detection and quantification of multiple metabolites, including acetyl-CoA, in a complex biological matrix.
At Creative Proteomics, we offer comprehensive LC-MS-based metabolomics services for a wide range of biological samples, including tissues, cells, and biofluids. Our LC-MS platform utilizes state-of-the-art instruments and software, coupled with advanced sample preparation techniques, to achieve high sensitivity, specificity, and reproducibility.
To analyze acetyl-CoA, we typically extract metabolites from the biological samples using organic solvents or aqueous solutions, followed by derivatization with a suitable reagent, such as N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA). This derivatization step enhances the volatility and stability of acetyl-CoA, enabling its efficient detection and quantification by LC-MS.
We separate the derivatized metabolites by reversed-phase liquid chromatography (RPLC) using a C18 column, which allows for the separation of hydrophobic metabolites based on their polarity and hydrophobicity. After separation, the metabolites are ionized by electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI), and the resulting ions are analyzed by mass spectrometry in positive or negative ion mode.
The LC-MS data are then processed using specialized software, such as XCMS or MZmine, which allows for peak detection, alignment, and quantification of metabolites. We use high-quality standards and internal controls to ensure the accuracy and reproducibility of the data.
Using this approach, we have successfully quantified acetyl-CoA levels and metabolic fluxes in various biological samples, including cancer cells, liver tissues, and blood plasma. Our LC-MS-based acetyl-CoA analysis has provided valuable insights into the regulation of cellular metabolism and its perturbation in diseases.
Sample Requirements for Acetyl-CoA Assay
| Sample Requirements | Description |
|---|---|
| Sample Type | Biological samples containing acetyl-CoA, such as cell lysates, tissue homogenates, blood plasma, or urine. |
| Sample Collection | Collect samples using appropriate protocols. For example, collect blood plasma using EDTA as an anticoagulant to prevent coagulation. Urine samples can be collected in sterile containers. |
| Sample Preparation | Homogenize or lyse the samples using suitable buffers or solvents to release intracellular contents, including acetyl-CoA. Commonly used buffers include phosphate buffer or Tris-HCl buffer. |
| Sample Extraction | Consider using organic solvents such as methanol or acetonitrile to extract and isolate acetyl-CoA from the sample matrix. Optimization of extraction conditions may be required. |
| Sample Storage | Store samples at ultra-low temperatures, typically at -80°C, to minimize degradation until analysis. Avoid repeated freeze-thaw cycles as they can lead to sample degradation. |
| Internal Standard | Prepare or select a suitable internal standard for accurate quantification of acetyl-CoA. Commonly used internal standards include stable isotope-labeled acetyl-CoA. |
Feedback to Customers
Creative Proteomics will provide you with detailed technical reports, including
- Experimental steps
- Related mass spectrometry parameters
- Part of the mass spectrum picture
- Raw data
- Metabolic molecular identification results
Creative Proteomics offers several approaches to metabolomics studies, delivers precise and detailed data and analysis report. We can also customize the methods or establish new methods together with our collaborators, so they are fit-for-purpose and meet your specific needs. If you have any questions or specific requirements, please feel free to contact us.
References
- Riera-Borrull M, Rodríguez-Gallego E, Hernández-Aguilera A, et al. Exploring the process of energy generation in pathophysiology by targeted metabolomics: performance of a simple and quantitative method. Journal of the American Society for Mass Spectrometry, 2015, 27(1): 168-177.
- Kim M J, Lee M Y, Shon J C, et al. Untargeted and targeted metabolomics analyses of blackberries – Understanding postharvest red drupelet disorder. Food Chemistry, 2019, 300:125169.
- Wang X, Zhao X, Zhao J, et al. Serum metabolite signatures of epithelial ovarian cancer based on targeted metabolomics. Clinica Chimica Acta, 2021, 518: 59-69.