Monoamine Analysis Service
Submit Your Inquiry- Service Details
- Case and Demo
- FAQ
- Publications
What is Monoamine?
Monoamines are a class of biologically active compounds derived from aromatic amino acids through decarboxylation, including well-known neurotransmitters such as dopamine, serotonin (5-HT), norepinephrine, and histamine. These small, nitrogen-containing molecules regulate critical physiological processes in the nervous system, immune function, gastrointestinal motility, and even microbial fermentation pathways.
In biomedical research, monoamines are essential targets for understanding neurological disorders, psychiatric diseases, and the gut–brain axis. In industrial biotechnology, their metabolic intermediates serve as biomarkers for optimizing strain engineering, fermentation yield, and product safety in food and beverage production.
Advantages of Monoamine Analysis Service
- Sub-Nanomolar Sensitivity – Limits of detection reach 0.05 ng mL⁻¹ (~0.3 nM) for dopamine, serotonin, and related analytes, enabling trace-level studies in small animal models or low-volume clinical samples.
- Five-Order Dynamic Range – Linear quantitation from 0.05 ng mL⁻¹ up to 50 µg mL⁻¹ captures both basal neurotransmitter levels and pharmacologically elevated concentrations in a single run.
- Ultra-Low Variability – Intra-batch precision is ≤ 5 % RSD, while inter-batch precision is ≤ 8 % RSD across 20+ monoamines, safeguarding longitudinal studies.
- High Throughput Capacity – Up to 192 samples processed and reported every 24 hours on a dual-column UHPLC-MS/MS system, shortening large-cohort timelines.
- Robust Extraction Efficiency – Solid-phase or protein-precipitation workflows deliver 85–110 % recovery (n = 6 spike-ins per matrix), minimizing data correction for losses.
- Minimal Matrix Interference – Optimized clean-up keeps ion-suppression or enhancement to < 10 %, ensuring reliable quantitation even in lipid-rich or salt-heavy samples.
- Rapid Turnaround – Standard projects (≤ 96 samples) ship results in 5–7 business days from sample receipt; expedited 48-hour reporting is available for time-critical studies.
- Accurate Calibration – Eight-point calibration curves with R² ≥ 0.995 for every analyte guarantee quantitative fidelity across the full range.
- Micro-Volume Compatibility – Proprietary micro-extraction protocol quantifies monoamines from as little as 50 µL plasma/CSF or 10 mg tissue, preserving precious specimens.
Monoamine Analysis Service Offered by Creative Proteomics
At Creative Proteomics, we offer a comprehensive suite of monoamine analysis services tailored to the diverse needs of biomedical researchers, fermentation specialists, and industrial R&D teams. Our service portfolio includes:
- Quantitative Monoamine Profiling: Using isotope-dilution LC-MS/MS, we provide absolute quantification of major monoamines—including dopamine, serotonin, norepinephrine, epinephrine, and histamine—in various biological and industrial matrices.
- Monoamine Metabolite Mapping: We analyze key downstream metabolites such as 5-HIAA, HVA, DOPAC, and MHPG, offering insights into neurotransmitter catabolism and turnover dynamics in vivo or in vitro.
- Trace Amine Detection: Our extended panels cover trace biogenic amines like tyramine, tryptamine, octopamine, and β-phenylethylamine, which are relevant in neuromodulation and microbial metabolic studies.
- Biogenic Amine Screening for Fermented Products: We offer safety-focused analysis of histamine, cadaverine, putrescine, and related amines in food and beverage matrices to ensure compliance with quality and regulatory standards.
- Custom Panel Design and Expansion: Projects requiring specialized detection of uncommon monoamines or synthetic analogs (e.g., drug metabolites, dietary supplements) can benefit from our customizable assay development services.
- Kinetic and Time-Course Sampling Support: For flux studies and pathway modeling, we support time-resolved sampling protocols ranging from minutes to hours post-treatment, allowing dynamic monitoring of monoamine changes.
- Pathway-Level Data Annotation and Visualization: Beyond raw concentration data, we offer pathway-based interpretation, mapping monoamine fluctuations onto curated metabolic networks (e.g., KEGG, Reactome) to facilitate biological insight.
Each project is fully customizable in terms of target analytes, matrices, and reporting depth—making our monoamine analysis services suitable for both discovery-driven research and process-controlled industrial applications.
List of Detected Monoamine and Related Metabolites
| Category | Analyte Name | Representative Abbreviation | Related Metabolic Pathway |
|---|---|---|---|
| Catecholamines | Dopamine | DA | Tyrosine metabolism, dopaminergic synapse |
| Norepinephrine | NE | Catecholamine biosynthesis | |
| Epinephrine | EPI | Adrenergic signaling pathway | |
| 3,4-Dihydroxyphenylacetic acid | DOPAC | Dopamine degradation | |
| Homovanillic acid | HVA | Dopamine degradation | |
| 3-Methoxytyramine | 3-MT | Dopamine metabolism | |
| 3-Methoxy-4-hydroxyphenylglycol | MHPG | NE/EPI metabolism | |
| Indolamines | Serotonin | 5-HT | Tryptophan metabolism, serotonergic synapse |
| 5-Hydroxytryptophan | 5-HTP | Serotonin biosynthesis | |
| 5-Hydroxyindoleacetic acid | 5-HIAA | Serotonin degradation | |
| Histamine Pathway | Histamine | HIS | Histidine metabolism, inflammatory mediator |
| N-Methylhistamine | MeHIS | Histamine methylation | |
| Imidazoleacetic acid | IAA | Histamine catabolism | |
| Trace Amines | Tyramine | TYR | Tyrosine decarboxylation |
| Tryptamine | TRYP | Tryptophan decarboxylation | |
| Octopamine | OCT | Tyramine hydroxylation | |
| β-Phenylethylamine | PEA | Phenylalanine decarboxylation | |
| Food/Fermentation | Putrescine | PUT | Arginine, ornithine decarboxylation |
| Cadaverine | CAD | Lysine decarboxylation | |
| Agmatine | AGM | Arginine decarboxylation | |
| Ethanolamine | ETA | Phospholipid metabolism | |
| Others / Custom | Salsolinol | SAL | DA-derived neurotoxic metabolite (optional) |
| Normetanephrine | NMN | NE methylation | |
| Metanephrine | MN | EPI methylation | |
| Melatonin | MEL | Tryptophan → Serotonin → Melatonin pathway |
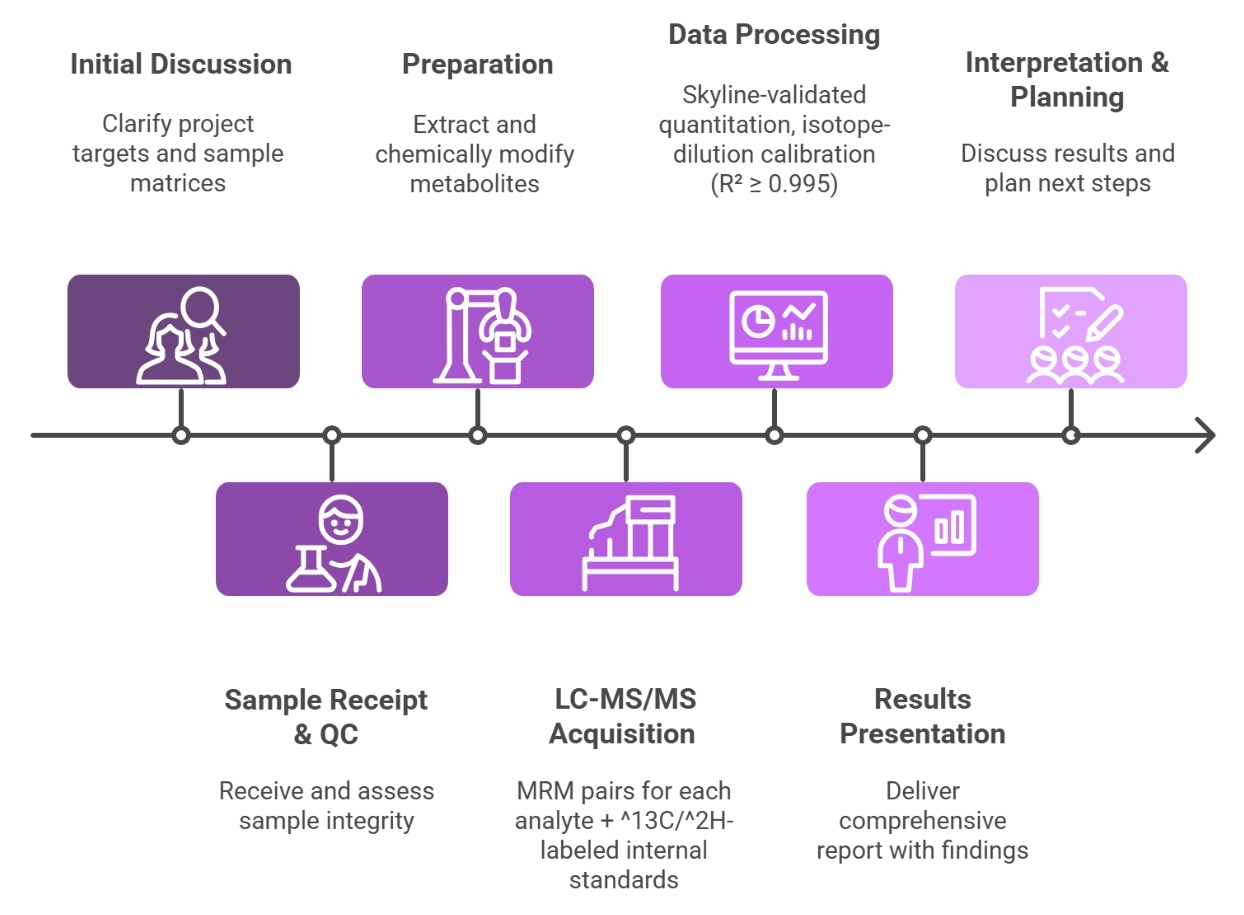
Workflow for Monoamine Analysis Service
Technology Platform for Monoamine Analysis Service
LC-MS/MS – Our primary technique using SCIEX QTRAP® 6500+ and Thermo TSQ Altis™ for ultra-sensitive, targeted analysis of polyamines and their derivatives.
GC-MS – Ideal for volatile or derivatized amines, performed on Agilent 7890B-5977A systems with high resolution and reproducibility.
HPLC – Quantification after derivatization using the Agilent 1260 Infinity II HPLC system, ensuring reliable separation and detection.



SCIEX Triple Quad™ 6500+ (Figure from Sciex)
Agilent 7890B-5977A (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Monoamine Analysis Service
| Sample Type | Minimum Volume / Amount | Notes |
|---|---|---|
| Plasma / Serum | 200 μL | EDTA or heparin tubes preferred; avoid hemolysis |
| Cerebrospinal Fluid (CSF) | 100 μL | Must be collected in sterile polypropylene tubes |
| Tissue (brain, liver, etc) | 50 mg | Snap-frozen in liquid nitrogen; store at –80°C |
| Cell Pellet | ≥ 1 × 10⁶ cells | Washed with PBS and frozen dry |
| Fermentation Broth | 1–2 mL | Filtered or clarified; sterile tubes recommended |
| Urine | 1 mL | First morning void preferred; collect in sterile containers |
| Food/Beverage | 2 mL or 2 g | Homogenized; no additives or preservatives |
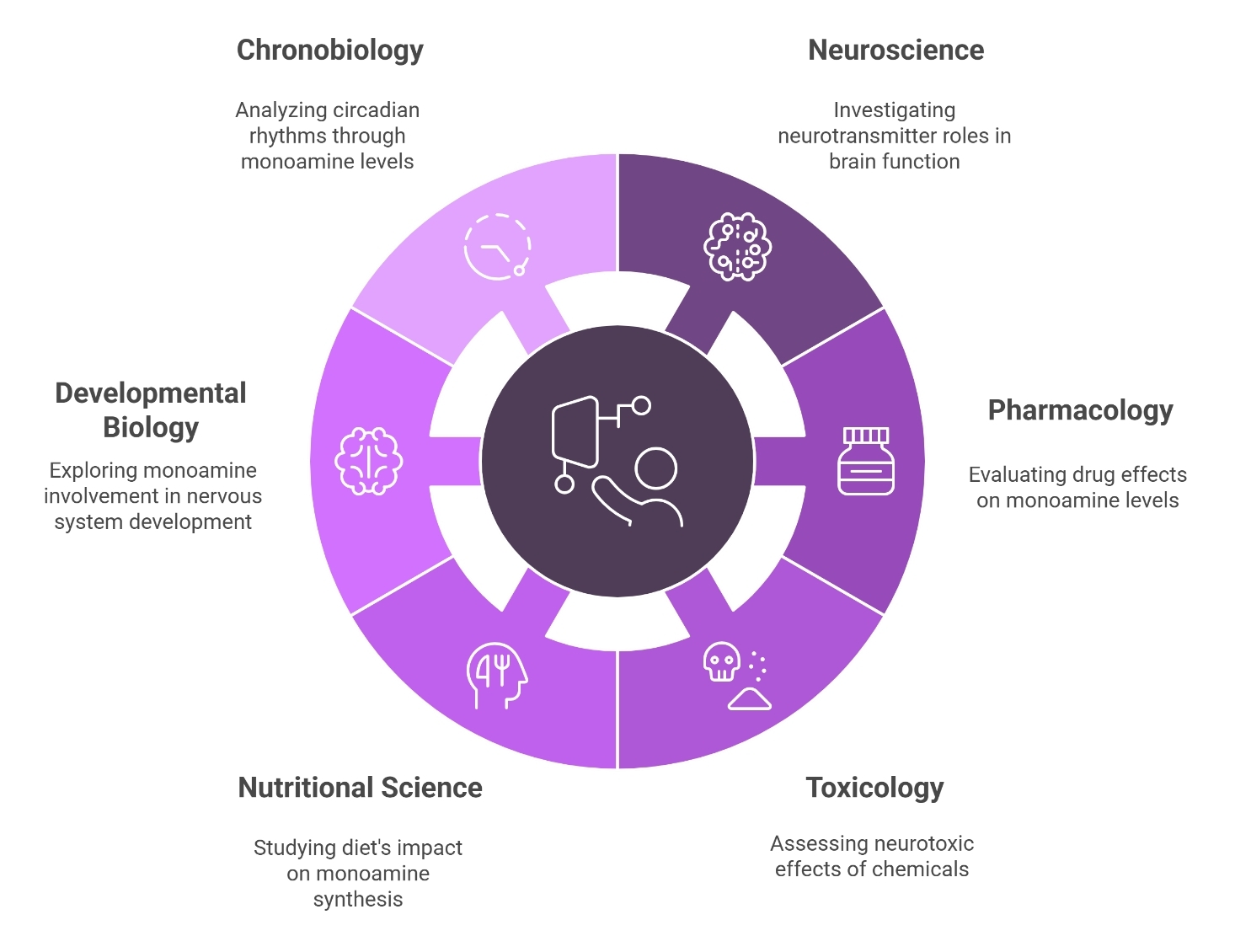
Applications of Monoamine Assay Service
An LC-MS/MS method for simultaneous analysis of up to six monoamines from brain tissues
Journal: Journal of Chromatography B
Published: 2023
Background
Monoamines are critical neuromodulators involved in regulating various physiological functions such as mood, movement, sleep, and cognition across species. Traditional analytical methods for measuring monoamines in brain tissues—such as HPLC-EC—face limitations in sensitivity and specificity, especially when dealing with low-abundance analytes. Existing techniques often focus on only dopamine and serotonin and are typically semi-quantitative. To overcome these limitations, the study presents a novel LC-MS/MS method using a Triple Quadrupole Mass Spectrometer to accurately and simultaneously quantify six monoamines—dopamine, serotonin, octopamine, tyramine, melatonin, and N-acetylserotonin—from small brain tissue samples of Drosophila and mice.
Methods
Reference standards and deuterated internal standards of six monoamines were sourced from certified suppliers. Brain tissues from Drosophila and mice were dissected, homogenized in 0.1% formic acid, centrifuged, and supernatants stored at −80°C. Stock solutions were prepared in ethanol, diluted to working and spiking solutions for calibration and system tuning. LC-MS/MS analysis utilized a Thermo Scientific Quantiva triple quadrupole mass spectrometer with heated electrospray ionization, coupled to an UltiMate 3000 UHPLC system. Chromatography was performed on a C-18 column using gradient elution with formic acid and acetonitrile. Selected reaction monitoring in positive ion mode allowed sensitive and specific detection of dopamine, serotonin, octopamine, tyramine, melatonin, and N-acetylserotonin with optimized mass transitions and collision energies. Calibration curves ranged from 0.25 to 10 ng/mL, showing high linearity and reproducibility. Extraction recovery was above 85% for all analytes.
What Creative Proteomics can provide:
Creative Proteomics can offer customized LC-MS/MS method development and validation for multi-amine quantification, precise sample preparation protocols, high-sensitivity biomolecule detection, quantitative data analysis, and comprehensive reporting tailored to monoamine profiling in biological tissues.
Results
Development of LC-MS/MS Method:
- A method was developed to measure six monoamines (DA, 5-HT, OA, TA, MT, NAS) simultaneously in a single analytical run using gradient reverse-phase chromatography coupled with mass spectrometry.
- Analytical conditions were optimized using synthetic standards with corresponding deuterated internal standards.
- Adequate chromatographic separation was achieved for all six monoamines, with specific retention times identified for each compound.
- Enantiomers of DA, OA, and TA were not chromatographically separated.
Detection in Drosophila Brain Extracts:
- All six monoamines were detected in adult fly brain extracts.
- OA and MT were found at low levels, with NAS showing potential fragmentation affecting its abundance measurement.
- Chromatographic retention times matched those observed with standards.
Quantification in Drosophila Brain:
- Standard curves were linear over a range of 0.25 to 10 ng/mL, with a limit of quantification at 0.25 ng/mL.
- DA, 5-HT, TA, and NAS were detected at quantifiable levels.
- OA was detected but at low levels making absolute quantification difficult; thus, only relative levels were reported.
Detection and Quantification in Mouse Brain Extracts:
- Four monoamines (DA, 5-HT, TA, MT) were measured in mouse cortex extracts.
- Using larger sample volumes (500 µL) improved detection and quantification, including MT.
- OA and NAS were not measured in mouse brain as OA is insect-specific.
Technical Considerations:
- Ion suppression and peak distortion issues for highly polar monoamines (DA, OA, TA) were mitigated by using a divert valve to reduce flow to the mass spectrometer during early elution.
- Non-polar monoamines (5-HT, MT, NAS) showed stable retention and peak symmetry, enabling consistent quantification.
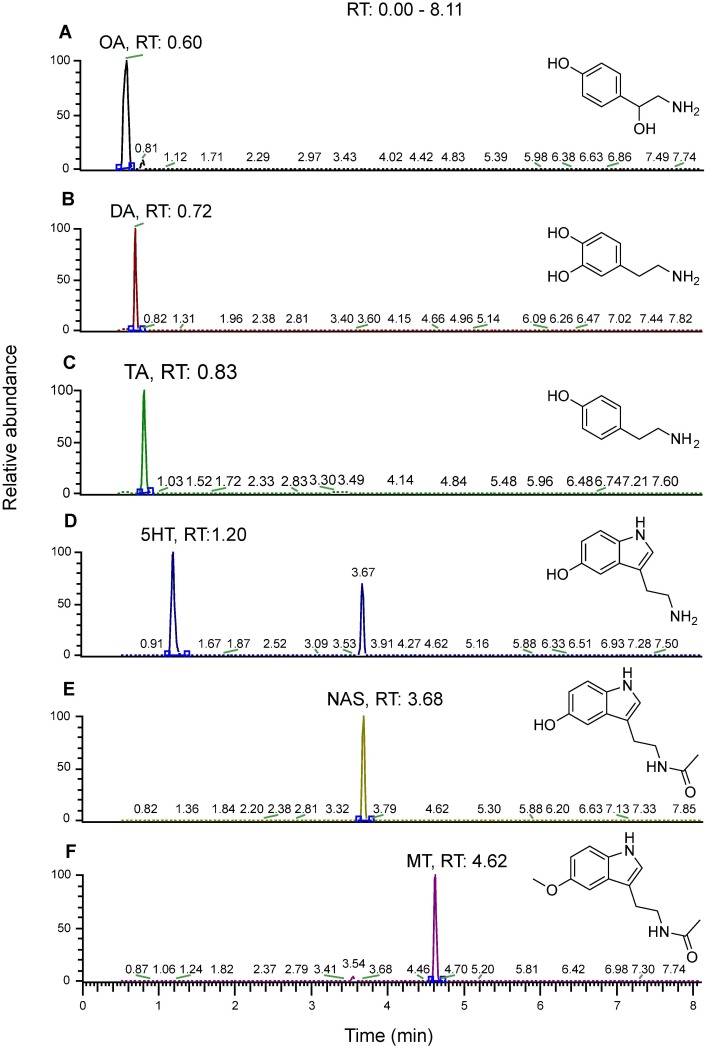 Mass spectrometry SRM chromatograms of six monoamine standards (10 ng/mL), showing transitions and structures: (A) OA, (B) DA, (C) TA, (D) 5-HT, (E) NAS, (F) MT.
Mass spectrometry SRM chromatograms of six monoamine standards (10 ng/mL), showing transitions and structures: (A) OA, (B) DA, (C) TA, (D) 5-HT, (E) NAS, (F) MT.
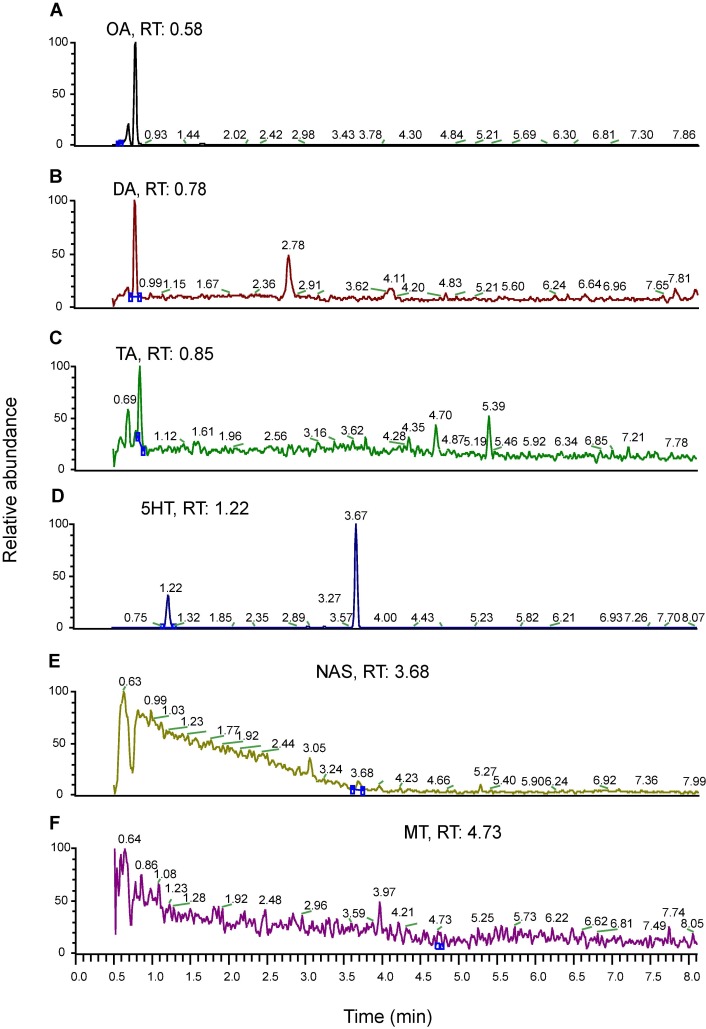 Representative traces for mass spectrometric analysis of monoamines derived from brain lysates.
Representative traces for mass spectrometric analysis of monoamines derived from brain lysates.
Reference
- Davla, Sejal, et al. "An LC-MS/MS method for simultaneous analysis of up to six monoamines from brain tissues." Journal of Chromatography B 1216 (2023): 123604.
What are the typical limits of detection (LOD) and quantification (LOQ) you achieve?
We routinely reach LODs of 0 .05–0 .1 ng mL⁻¹ and LOQs of 0 .2–0 .3 ng mL⁻¹ across most biofluids and tissue extracts; the exact performance for your matrix appears in the batch-specific QC report.
I only have micro-samples—what is the absolute minimum volume or mass you can process?
With our micro-extraction protocol we can work with as little as 50 µL of plasma/CSF or 10 mg of tissue. Let us know in advance so we can reserve low-dead-volume consumables.
Can you handle high-salt or lipid-rich matrices such as artificial-buffered CSF or adipose tissue?
Yes. A salt-tolerant solid-phase-extraction cleanup and dedicated delipidation step remove ≥ 95 % of interfering compounds while preserving monoamines.
Do you normalise urinary monoamine concentrations to creatinine?
Creatinine normalisation is optional. Simply include an extra 200 µL aliquot and we'll run an enzymatic creatinine assay, delivering both raw and creatinine-adjusted values.
How many biological replicates do I need for robust statistics?
For typical group comparisons we recommend at least six biological replicates per group. Power modelling at α = 0.05 shows this detects ~30 % fold-changes with 80 % power.
In what file formats will I receive the results?
You'll receive:
- A concentration matrix in both Excel and CSV
- Annotated chromatograms in PDF
- Raw vendor files (.wiff or .d) for your own re-analysis
A secure download link remains active for 30 days.
Can the monoamine data be integrated with existing metabolomics or proteomics datasets?
Absolutely. We supply a sample × analyte matrix compatible with MetaboAnalyst, Ingenuity Pathway Analysis, and common R/Bioconductor pipelines, making cross-omics correlation straightforward.
How stable are monoamines during transit if shipping takes more than 48 hours?
Controlled studies show ≤ 4 % degradation for dopamine, serotonin, and histamine when samples remain below –60 °C on dry ice for up to 72 hours. For longer routes we recommend liquid-nitrogen shippers or our pre-charged phase-change containers that maintain ≤ –70 °C for five days.
How many freeze–thaw cycles can the extracts tolerate?
Pilot testing indicates concentration drift remains < 5 % across two freeze–thaw cycles. We advise aliquoting upon receipt and avoiding more than one additional cycle to keep total RSD under 6 %.
What internal standards are used and how many QC checkpoints are in each run?
Every analyte is paired with a ^13C- or ^2H-labelled analogue; two pooled QC samples, one process blank, and one system suitability standard are injected every 20 unknowns, giving a QC frequency of 15 %.
High Levels of Oxidative Stress Early after HSCT Are Associated with Later Adverse Outcomes
Cook, E., Langenberg, L., Luebbering, N., Ibrahimova, A., Sabulski, A., Lake, K. E., ... & Davies, S. M.
Journal: Transplantation and Cellular Therapy
Year: 2024
Multiomics of a rice population identifies genes and genomic regions that bestow low glycemic index and high protein content
Badoni, S., Pasion-Uy, E. A., Kor, S., Kim, S. R., Tiozon Jr, R. N., Misra, G., ... & Sreenivasulu, N.
Journal: Proceedings of the National Academy of Sciences
Year: 2024
The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner
Norman, J. E., Milenkovic, D., Nuthikattu, S., & Villablanca, A. C.
Journal: International Journal of Molecular Sciences
Year: 2024
UDP-Glucose/P2Y14 Receptor Signaling Exacerbates Neuronal Apoptosis After Subarachnoid Hemorrhage in Rats
Kanamaru, H., Zhu, S., Dong, S., Takemoto, Y., Huang, L., Sherchan, P., ... & Zhang, J. H.
Journal: Stroke
Year: 2024
Pan-lysyl oxidase inhibition disrupts fibroinflammatory tumor stroma, rendering cholangiocarcinoma susceptible to chemotherapy
Burchard, P. R., Ruffolo, L. I., Ullman, N. A., Dale, B. S., Dave, Y. A., Hilty, B. K., ... & Hernandez-Alejandro, R.
Journal: Hepatology Communications
Year: 2024
Comparative metabolite profiling of salt sensitive Oryza sativa and the halophytic wild rice Oryza coarctata under salt stress
Tamanna, N., Mojumder, A., Azim, T., Iqbal, M. I., Alam, M. N. U., Rahman, A., & Seraj, Z. I.
Journal: Plant‐Environment Interactions
Year: 2024
Teriflunomide/leflunomide synergize with chemotherapeutics by decreasing mitochondrial fragmentation via DRP1 in SCLC
Mirzapoiazova, T., Tseng, L., Mambetsariev, B., Li, H., Lou, C. H., Pozhitkov, A., ... & Salgia, R.
Journal: iScience
Year: 2024
Physiological, transcriptomic and metabolomic insights of three extremophyte woody species living in the multi-stress environment of the Atacama Desert
Gajardo, H. A., Morales, M., Larama, G., Luengo-Escobar, A., López, D., Machado, M., ... & Bravo, L. A.
Journal: Planta
Year: 2024
A personalized probabilistic approach to ovarian cancer diagnostics
Ban, D., Housley, S. N., Matyunina, L. V., McDonald, L. D., Bae-Jump, V. L., Benigno, B. B., ... & McDonald, J. F.
Journal: Gynecologic Oncology
Year: 2024
Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice
Chargo, N. J., Neugebauer, K., Guzior, D. V., Quinn, R. A., Parameswaran, N., & McCabe, L. R.
Journal: Frontiers in Cell and Developmental Biology
Year: 2024
Proteolytic activation of fatty acid synthase signals pan-stress resolution
Wei, H., Weaver, Y. M., Yang, C., Zhang, Y., Hu, G., Karner, C. M., ... & Weaver, B. P.
Journal: Nature Metabolism
Year: 2024