Antibiotic Residue Analysis Overview: What It Is and Why It Matters
Antibiotic residues are trace amounts of antimicrobial drugs and their metabolites that remain in food products, environmental compartments or biological samples after administration or discharge. In real-world scenarios, multiple antibiotic classes—such as sulfonamides, tetracyclines, macrolides, fluoroquinolones and β-lactams—often co-exist in a single sample, and concentrations can be at sub-ppb to ng·L⁻¹ levels.
Antibiotic residue analysis with LC–MS/MS is a targeted, quantitative approach to measure trace levels of multiple antibiotics and their key metabolites in complex samples. Instead of screening one compound at a time, a single method can track many drugs across large sample sets with high sensitivity and specificity.
You typically need this service when:
- You must prove or quantify antibiotic presence in food, environmental or biological samples.
- You need numeric concentration data, not just "detected / not detected".
- You want a multi-class, multi-sample study without spending months on method development and validation.
Antibiotic Residue Panels and Typical Study Designs
Multi-Class Residue Panels
We provide multi-class antibiotic residue panels that quantify several major antibiotic classes in a single LC–MS/MS run. These panels are ideal for:
- Screening large sample sets
- Comparing treatments, time points or locations
- Generating consistent, numeric data for downstream risk or mechanistic analysis
Each panel is pre-optimised for coverage, sensitivity and throughput.
Matrix-Specific Panels
To ensure data integrity, we offer tailored extraction and cleanup protocols designed to minimize matrix effects and guarantee precise quantification for specific sample types:
- Food Safety Panel: Covers animal- and plant-derived matrices, including meat, offal, dairy, eggs, aquaculture products, honey, and produce.
- Environmental Panel: Designed for environmental monitoring across surface water, wastewater, groundwater, soil, and sediments.
- Biological Research Panel: Optimized for experimental biological samples such as plasma, serum, excreta, and tissue homogenates.
Custom Antibiotic Assays
For specialised applications, we can design custom antibiotic assays, for example:
- Panels focused on a single antibiotic class (e.g., only fluoroquinolones or only tetracyclines).
- Assays including metabolites, degradation products or transformation products of interest.
- Antibiotic residue modules integrated into broader targeted metabolomics projects, enabling you to analyse residues alongside host or microbial metabolites in one study.
Method Development & Validation
If your project involves unusual matrices or new molecules, we offer fit-for-purpose method development and validation, including:
- Selection and optimisation of extraction and clean-up strategies
- Tuning chromatographic conditions and MRM transitions
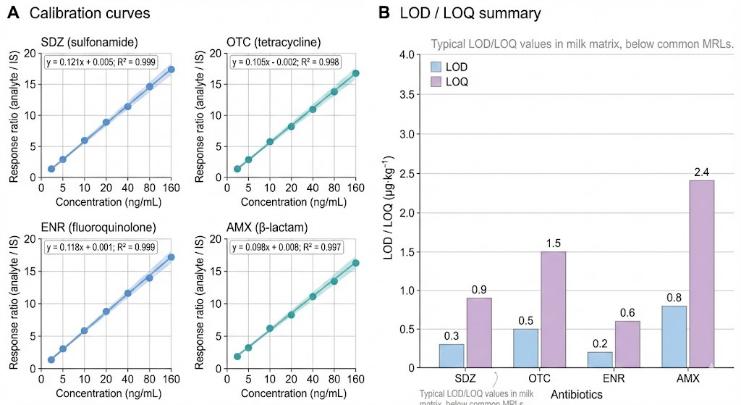
- Evaluation of LOD/LOQ, linearity, recovery, precision and matrix effects for your specific use case
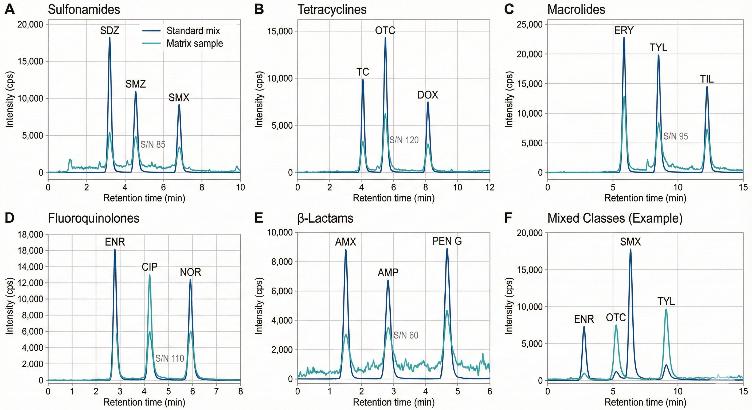
Target List: Antibiotic Classes and Analytes Covered
Our antibiotic residue LC–MS/MS panels are organized by major antibiotic classes. Below is an example of the typical analytes that can be included; the list can be tailored or expanded according to your study needs.
| Antibiotic Class |
Representative Analytes (Examples) |
Notes |
| Sulfonamides |
Sulfadiazine, Sulfamerazine, Sulfamethazine (Sulfadimidine), Sulfamethoxazole, Sulfadoxine, Sulfadimethoxine, Sulfamonomethoxine, Sulfachlorpyridazine, Sulfaquinoxaline |
Widely used in veterinary and human medicine; frequently monitored in food and environmental samples. |
| Tetracyclines |
Tetracycline, Oxytetracycline, Chlortetracycline, Doxycycline, Minocycline, Demeclocycline |
Common in livestock, aquaculture and clinical applications; prone to strong matrix effects in some samples. |
| Macrolides |
Erythromycin, Tylosin, Tilmicosin, Tulathromycin, Spiramycin, Josamycin, Azithromycin, Roxithromycin, Clarithromycin |
Important for food and water monitoring; often included in multi-residue panels. |
| Fluoroquinolones |
Enrofloxacin, Ciprofloxacin, Norfloxacin, Ofloxacin, Danofloxacin, Difloxacin, Marbofloxacin, Sarafloxacin, Levofloxacin |
Frequently used in veterinary and human therapy; relevant for residue and AMR-related studies. |
| β-Lactams |
Penicillin G, Penicillin V, Amoxicillin, Ampicillin, Cloxacillin, Oxacillin, Dicloxacillin, Ceftiofur, Cefalexin, Cefazolin |
Key penicillins and cephalosporins monitored in animal-derived foods and research samples. |
| Lincosamides & Amphenicols |
Lincomycin, Clindamycin, Chloramphenicol, Florfenicol, Thiamphenicol |
Included where relevant for regulatory or research purposes in food, feed or animal studies. |
| Selected Aminoglycosides / Others |
Gentamicin, Streptomycin, Neomycin, Kanamycin, Colistin (Polymyxin E) |
Available as part of extended or custom panels, depending on matrix and project goals. |
*The exact analyte list for your project (including additional antibiotics, metabolites and degradation products) can be provided as a detailed Excel or PDF file on request, and can be adjusted to match your internal target list or study protocol.
Advantages of Our Antibiotic Residue LC–MS/MS Service
- Multi-class coverage – Single LC–MS/MS method quantifies dozens of antibiotics across sulfonamides, tetracyclines, macrolides, fluoroquinolones, β-lactams and more in one run.
- High sensitivity – Detection limits typically reach sub-ppb levels in food matrices and ng·L⁻¹ ranges in water, suitable for trace-residue studies.
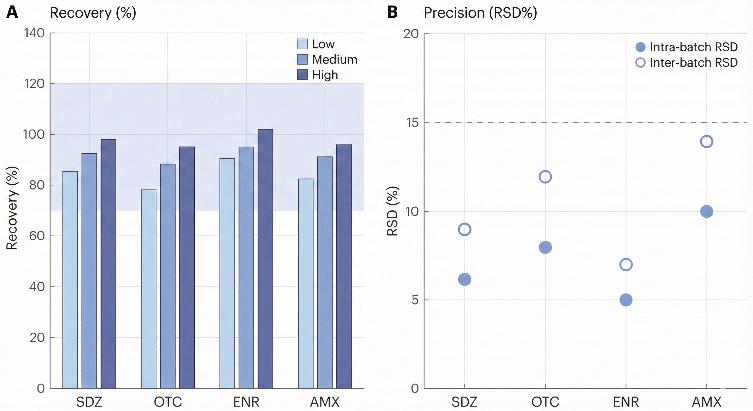
- Robust method performance – Validated workflows routinely achieve recoveries around 70–120% with intra-/inter-batch RSD commonly below 15%.
- High-precision calibration – Targeted LC–MS/MS with isotope-labeled internal standards; calibration curves typically reach R² ≥ 0.99 across the working range.
- Matrix-optimised extraction – SPE, LLE or QuEChERS-based clean-up is selected per matrix to control matrix effects and improve reproducibility.
- Scalable throughput – Batch designs support tens to hundreds of samples, enabling large surveillance studies, depletion trials and time-course experiments.
- Embedded quality control – Each batch includes blanks, matrix spikes, QC samples and system suitability checks to track method stability over time.
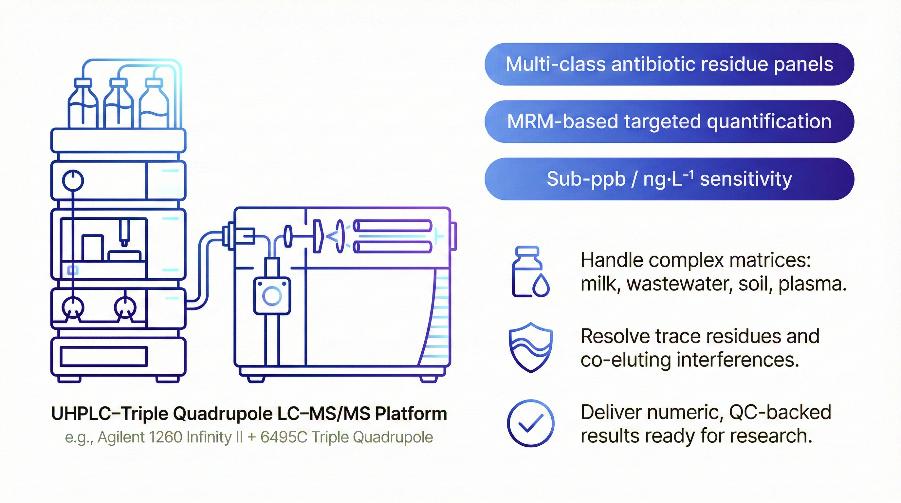
Analytical Platforms: LC–MS/MS Systems and Key Parameters
Our antibiotic residue analysis is performed on UHPLC systems coupled to triple quadrupole LC–MS/MS instruments, optimised for targeted, multi-residue quantification.
- UHPLC separation – Reversed-phase columns (e.g., C18, sub-3 µm), aqueous/organic gradients tuned for polar to moderately hydrophobic antibiotics, typical run time ~10–25 min per sample.
- Triple quadrupole MS – Electrospray ionisation (ESI), positive/negative polarity as required, operated in multiple reaction monitoring (MRM) to achieve high selectivity and sensitivity.
- High MRM capacity – Simultaneous monitoring of large MRM panels, enabling multi-class antibiotic quantification in a single injection.
- Stable long-run performance – Source and chromatographic conditions optimised for long sequences with complex matrices.
Where appropriate, GC–MS/MS can be used as a complementary platform for selected volatile or thermally stable antibiotics and degradation products, with dedicated extraction and derivatisation workflows.
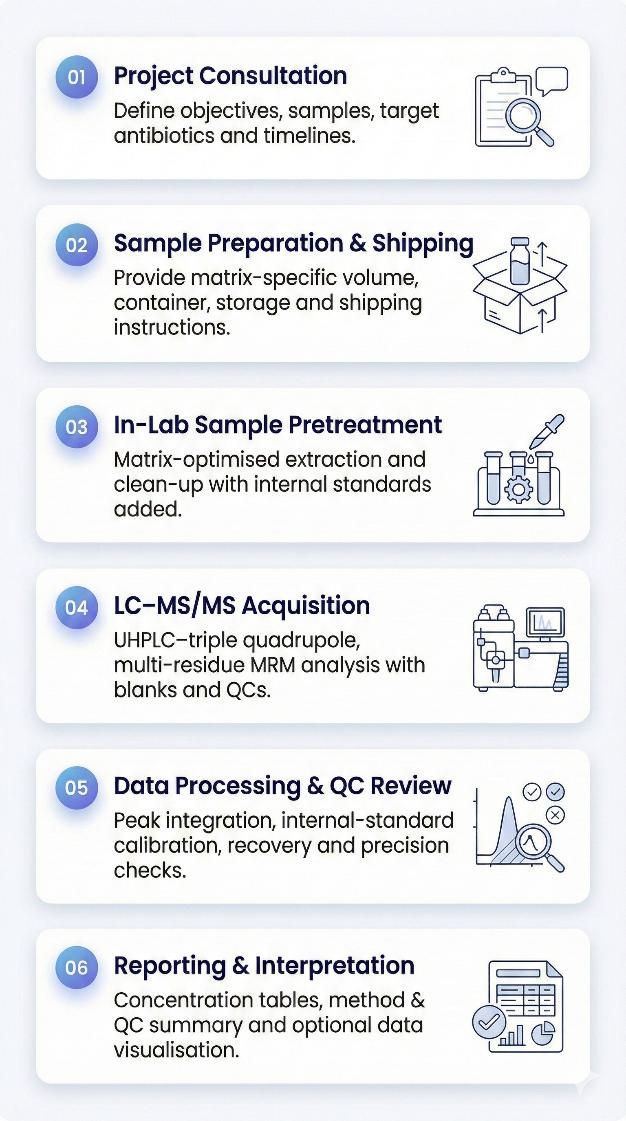
Workflow: How the Antibiotics Targeted Metabolomics Service Runs
Sample Requirements for Antibiotic Residue Analysis
| Sample Type |
Minimum Amount / Volume |
Container |
Storage |
Shipping |
Notes |
| Meat / Fish Tissue |
e.g. ≥ 5–10 g |
Pre-labelled tube/bag |
−20 °C or below |
Frozen, insulated |
Avoid repeated freeze–thaw cycles |
| Milk / Dairy |
e.g. ≥ 10–20 mL |
Polypropylene tube |
4 °C (short) / −20 °C (long) |
Cold packs / frozen |
Mix gently, avoid excessive foaming |
| Water (Surface) |
e.g. ≥ 250–500 mL |
Amber glass or HDPE bottle |
4 °C |
Cold packs |
Protect from light where relevant |
| Wastewater / Effluent |
e.g. ≥ 250–500 mL |
HDPE bottle |
4 °C |
Cold packs |
Note sampling time and location |
| Serum / Plasma |
e.g. ≥ 0.5–1 mL |
Cryovial |
−80 °C |
Dry ice |
Indicate anticoagulant if applicable |
| Urine / Feces |
e.g. ≥ 5–10 mL / 1–2 g |
Screw-cap tube |
−20 °C or below |
Frozen |
Homogenise feces if possible |
| Others (Custom) |
By agreement |
To be discussed |
As advised |
As advised |
Contact us for matrix-specific details |
Exact volumes, containers and conditions will be confirmed in your project quotation and sample submission guidelines.
Antibiotic Residue LC–MS/MS Results & Data Deliverables
- Quantitative result table (Excel/CSV) with antibiotic concentrations per sample, units, and <LOD/<LOQ flags.
- Summary method and QC report (PDF) describing sample preparation, LC–MS/MS method, calibration range and key performance metrics.
- QC overview table with recovery, precision (RSD) and basic validation parameters for the relevant matrix.
- Representative MRM chromatograms for selected antibiotics in standards and real samples.
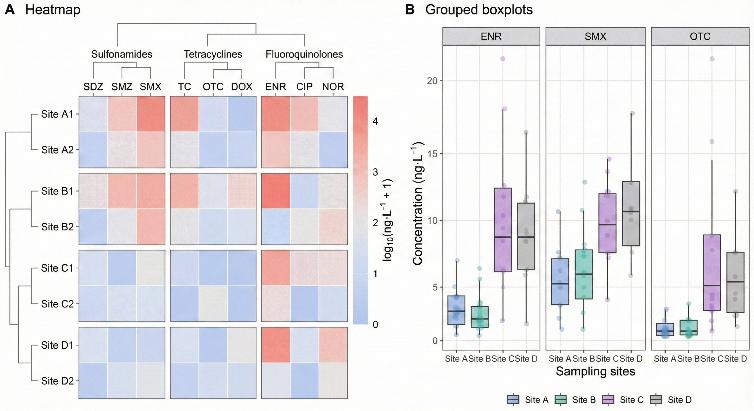
- Optional comparison plots (e.g., group-wise bar charts, boxplots or time-course curves) on request.
- Optional brief data interpretation notes aligned with the study design (e.g., between-group or time-point trends).
- Optional raw LC–MS/MS data files (vendor format) for in-house reprocessing or archiving.
Applications: Where Antibiotics Targeted Metabolomics Adds Value
Nutritional analysis of commercially available, complete plant- and meat-based dry dog foods in the UK
Brociek, R. A., Li, D., Broughton, R., & Gardner, D. S.
Journal: bioRxiv
Year: 2024
DOI: https://doi.org/10.1101/2024.09.11.612409
Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice
Chargo, N. J., Neugebauer, K., Guzior, D. V., Quinn, R. A., Parameswaran, N., & McCabe, L. R.
Journal: Frontiers in Cell and Developmental Biology
Year: 2024
DOI: https://doi.org/10.3389/fcell.2023.1324649
Elevated SLC7A2 expression is associated with an abnormal neuroinflammatory response and nitrosative stress in Huntington's disease
Gaudet, I. D., Xu, H., Gordon, E., Cannestro, G. A., Lu, M. L., & Wei, J.
Journal: Journal of Neuroinflammation
Year: 2024
DOI: https://doi.org/10.1186/s12974-024-03038-2
Enhance Trial: Effects of NAD3® on Hallmarks of Aging and Clinical Endpoints of Health in Middle Aged Adults: A Subset Analysis Focused on Blood Cell NAD⁺ Concentrations and Lipid Metabolism
Roberts, M. D., Osburn, S. C., Godwin, J. S., Ruple, B. A., La Monica, M. B., Raub, B. A., ... & Lopez, H. L.
Journal: Physiologia
Year: 2022
DOI: https://doi.org/10.3390/physiologia2010002
Inflammation primes the kidney for recovery by activating AZIN1 A-to-I editing
Heruye, S., Myslinski, J., Zeng, C., Zollman, A., Makino, S., Nanamatsu, A., ... & Hato, T.
Journal: bioRxiv
Year: 2023
DOI: https://doi.org/10.1101/2023.11.09.566426