Caryophyllene Analysis Service
Submit Your Inquiry- Service Details
- Case Study
What is Caryophyllene?
Caryophyllene is a natural bicyclic sesquiterpene that belongs to the class of compounds known as terpenes. It is found widely in nature, particularly in essential oils derived from various plants such as cloves, basil, hops, rosemary, and cannabis. Caryophyllene is notable for its spicy, woody aroma and is often used in perfumery and flavoring industries.
One unique characteristic of caryophyllene is its ability to act as a selective agonist of the cannabinoid receptor type 2 (CB2 receptor), which is part of the endocannabinoid system in mammals. This interaction gives caryophyllene potential therapeutic applications in areas related to inflammation, pain relief, and potentially other health benefits, similar to cannabinoids found in cannabis.
In addition to its pharmacological properties, caryophyllene is also studied for its antimicrobial, antioxidant, and anti-inflammatory effects, making it a subject of interest in both scientific research and commercial applications.
Caryophyllene Analysis by Creative Proteomics
Creative Proteomics offers a comprehensive suite of Caryophyllene Analysis services tailored to meet the specific needs of clients across various industries.
Caryophyllene Quantitative Analysis: Accurately measures caryophyllene concentration in a variety of sample types using high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) techniques. This service is ideal for essential oils, plant extracts, and natural products.
Caryophyllene Qualitative Analysis: Identifies and characterizes caryophyllene and its isomers using advanced chromatographic and spectroscopic methods. We confirm the presence of caryophyllene in complex matrices, providing crucial insights for product development and quality control.
Caryophyllene Purity Testing: Assesses the purity of caryophyllene in both raw materials and finished products, detecting impurities and related compounds. Our testing ensures compliance with industry standards, supporting regulatory requirements.
Caryophyllene Stability Studies: Evaluates the stability of caryophyllene under various conditions and monitors degradation products over time. This service aids in determining product shelf-life and optimizing storage conditions.
Profile Characterization: Provides comprehensive profiling of caryophyllene and related terpenes, offering detailed insights into terpene profiles across different sample types. This characterization is essential for research purposes and quality control.
Customized Analysis: Develops tailored analytical solutions to meet specific client needs, including customized protocols for unique sample requirements. We collaborate closely with clients to achieve desired analytical outcomes efficiently.
Regulatory Compliance Testing: Ensures caryophyllene content meets regulatory standards, preparing detailed reports for regulatory submissions and supporting compliance with international regulations.
Platforms for Caryophyllene Assay
High-Performance Liquid Chromatography (HPLC)
- Separates components based on their interaction with a stationary phase and a mobile phase.
- Provides high resolution and accurate quantification.
- Suitable for complex mixtures like essential oils and plant extracts.
Gas Chromatography-Mass Spectrometry (GC-MS)
- Separates components based on their volatility and analyzes them using mass spectrometry.
- Offers high sensitivity and specificity for identification of caryophyllene and its isomers.
- Ideal for volatile compounds in essential oils and cannabis extracts.
Gas Chromatography with Flame Ionization Detection (GC-FID)
- Separates and quantifies components based on their thermal conductivity.
- Provides quantitative analysis of caryophyllene with good sensitivity.
- Cost-effective for routine analysis in various samples.
Fourier Transform Infrared Spectroscopy (FTIR)
- Analyzes molecular vibrations to identify functional groups in samples.
- Rapid analysis suitable for screening purposes.
- Requires minimal sample preparation and provides qualitative information.
Liquid Chromatography-Mass Spectrometry (LC-MS)
- Separates and analyzes compounds based on their mass-to-charge ratio.
- Offers high sensitivity and specificity for quantification and identification.
- Useful for complex matrices and trace analysis in pharmaceutical and biological samples.
Sample Requirements of Caryophyllene Analysis
| Sample Type | Suggested Sample Amount |
|---|---|
| Essential Oils | 0.1 - 1 gram |
| Plant Extracts | 0.1 - 1 gram |
| Cannabis Extracts | 0.1 - 0.5 gram |
| Herbal Products | 0.5 - 2 grams |
| Food and Beverage Products | 1 - 5 grams |
| Pharmaceutical Preparations | 0.5 - 2 grams |
| Cosmetic Formulations | 0.5 - 2 grams |
| Raw Materials | 0.5 - 2 grams |
Deliverables for Caryophyllene Analysis
Quantitative Analysis Report: Detailed quantification of caryophyllene content in your sample, including concentrations measured using HPLC or GC-MS, along with essential data such as peak areas, retention times, and calculated concentrations.
Qualitative Analysis Report: Identification and characterization of caryophyllene and its isomers, supported by spectral data confirming the presence of caryophyllene and comparative analysis with reference standards for validation.
Purity Testing Report: Evaluation of caryophyllene purity, including identification and quantification of impurities, and compliance assessment against relevant standards.
Customized Analysis Report (if applicable): Tailored solutions based on your specific requirements, including custom protocols, methodologies, and interpretations of results.
Title
Optimization and Validation of HPLC Methodology for the Analysis of C. verbenacea Essential Oil
Background
This study focuses on the development and optimization of a High-Performance Liquid Chromatography (HPLC) method for analyzing the essential oil of Cordia verbenacea, specifically targeting its main markers, carvacrol (CAR) and humulene (HUM). The aim was to achieve an ideal separation with accurate quantification, ensuring the method's applicability to commercial formulations like creams and aerosols.
Samples
The samples analyzed included essential oil from C. verbenacea, as well as commercial pharmaceutical formulations containing this essential oil in the form of aerosols and creams.
Technical Methods
Optimization of Chromatographic Conditions: The Box–Behnken experimental design was utilized to determine the optimal conditions. Mid-range settings were found ideal, with:
- Flow rate: 1 ml/min
- Column temperature: 40°C
- HP-β-CD concentration: 20 mM
- Solvent composition: 8% methanol, 51% HP-β-CD in aqueous solution (pH 3.0), 41% acetonitrile
- Column: C4
Predicted and Experimental Values:
- Predicted resolution values: RCMP1-CAR of 2.05, RCAR-CMP2 of 1.69, and CAR retention time of 32.7 min.
- Experimental results: RCMP1-CAR of 1.81, RCAR-CMP2 of 1.66, and CAR retention time of 35.6 min.
Validation: The method was validated for linearity, selectivity, precision, accuracy, limit of detection (LD), and limit of quantification (LQ). The UV spectra and purity of chromatographic signals were confirmed with a similarity index higher than 0.9999. Linearity was proven with correlation coefficients (R²) over 0.99, and precision showed relative standard deviation (RSD) less than 5%.
Results
The developed method successfully separated and quantified CAR and HUM in C. verbenacea essential oil. The concentrations in commercial formulations (aerosols and creams) were close to the labeled amounts, demonstrating no significant interference from excipients. The resolution achieved was adequate, and the method's precision and accuracy were validated, ensuring reliable application in quality control and formulation studies. Specifically:
- Aerosols: HUM concentrations were 2.85% and 2.87%, CAR concentrations were 10.30% and 10.43%.
- Creams: HUM concentrations were 2.9%, CAR concentrations were 10.33% and 10.23%.
The method proved effective for both the identification and quantification of CAR and HUM, with CAR suggested as a marker for C. verbenacea essential oil due to its higher concentration relative to HUM.
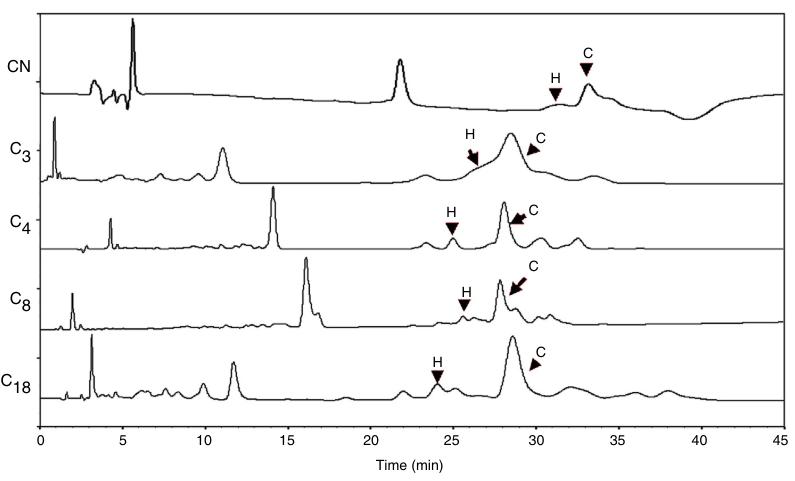 Representative chromatograms of the methods with the most adequate parameters, among the evaluated columns: CN (method 1), C3 (method 12), C4 (method 15), C8 (method 27) and C18 (method 29) at 210 nm. Legend: H. -humulene; C. trans-caryophyllene.
Representative chromatograms of the methods with the most adequate parameters, among the evaluated columns: CN (method 1), C3 (method 12), C4 (method 15), C8 (method 27) and C18 (method 29) at 210 nm. Legend: H. -humulene; C. trans-caryophyllene.
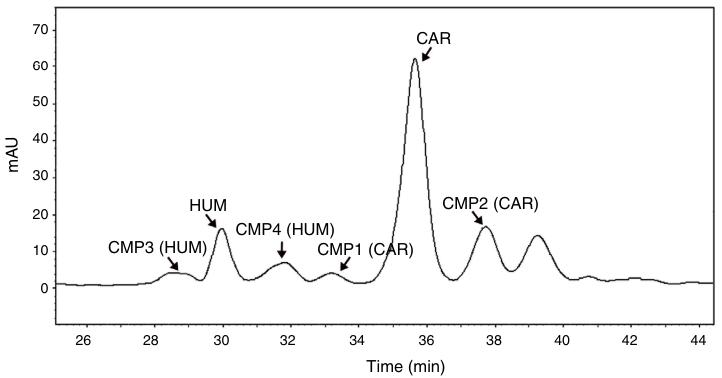 HPLC Chromatogram obtained for the optimized conditions. HUM. -humulene; CAR. trans-caryophyllene; CMP. unidentified component.
HPLC Chromatogram obtained for the optimized conditions. HUM. -humulene; CAR. trans-caryophyllene; CMP. unidentified component.
Reference
- da Gomes, Márcio Vinícius Silva, et al. "Development and validation of a quantification method for α-humulene and trans-caryophyllene in Cordia verbenacea by high performance liquid chromatography." Revista Brasileira de Farmacognosia 29.2 (2019): 182-190.