Mucin Type of O-glycan Analysis Service
Submit Your InquiryOverview
Protein glycosylation is an important post-translational modification. The research on protein glycosylation contribute to explain the diverse function of protein and its significant role in many physiological and pathological processes. Glycosylation of cell membrane surface proteins and secretory proteins mainly includes O-type glycosylation and N-type glycosylation. At present, little is known about the biological function of mucin type of O-glycosylation in vivo, so that the research on the physiological function of mucin type of O-glycan in vivo and its correlation with diseases will be the priority of Glycometabolomics development in the future.
There are mainly two core types of O-mucin glycosylation: core1-based core1 and core2 subtypes, and core3-based core3 and core4 subtypes, which are regulated by core1 galactosyltransferase (T-synthase) and core β1,3-GlcNAc-transferase (C3GnT), respectively. Core1 and core2 subtypes are the main types of O-type glycan, which are mainly expressed in vascular endothelial cells, epithelial cells and hematopoietic cells. The core3 and core4 subtypes were mainly expressed in intestinal epithelial cells.
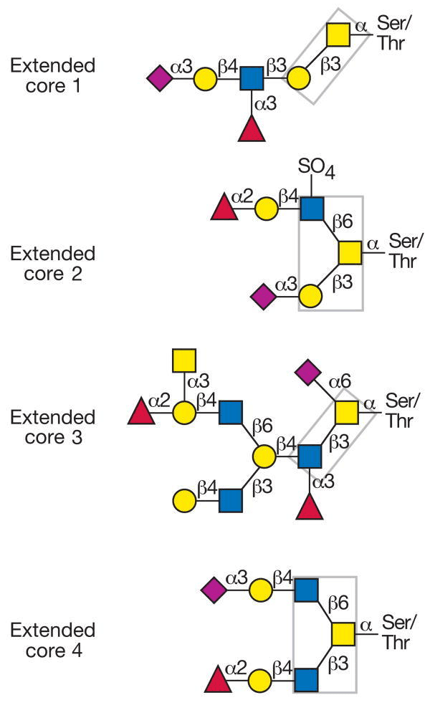 Fig 1. Common O-glycan core structures found on mucins.
Fig 1. Common O-glycan core structures found on mucins.
Advantage
- Short time-consuming
- High sensitivity and few detection restrictions
- High precision and good repeatability
- High throughput
- Customized service
Experimental process
O-glycan modification analysis is an effective method to identify glycan linkage binding sites. The principle is that stable substituents are introduced into each free hydroxyl group of natural polysaccharides, and then glycosidic bonds with much weaker stability than ether are cleaved by acid hydrolysis. As a result, a single methylated monosaccharide with free hydroxyl group is produced at the previously introduced link site. Partially methylated monosaccharides are opened together with reducing agents (usually boron deuterides) to introduce new hydroxyl groups (possibly deuterium atoms at Cmur1), which is helpful to identify the reducing end of each monosaccharide. Then all free hydroxyl groups were acetylated to form partially methylated partially methylated alditol acetates (PMAA), for follow-up analysis.
Service workflow
The Mucin Type of O-glycan was hydrolyzed from the glycoprotein, labeled with fluorescence, and then reacted with different glycosidases step by step. The products of each reaction were separated by hydrophilic chromatography and detected by fluorescence detector and mass spectrometer.
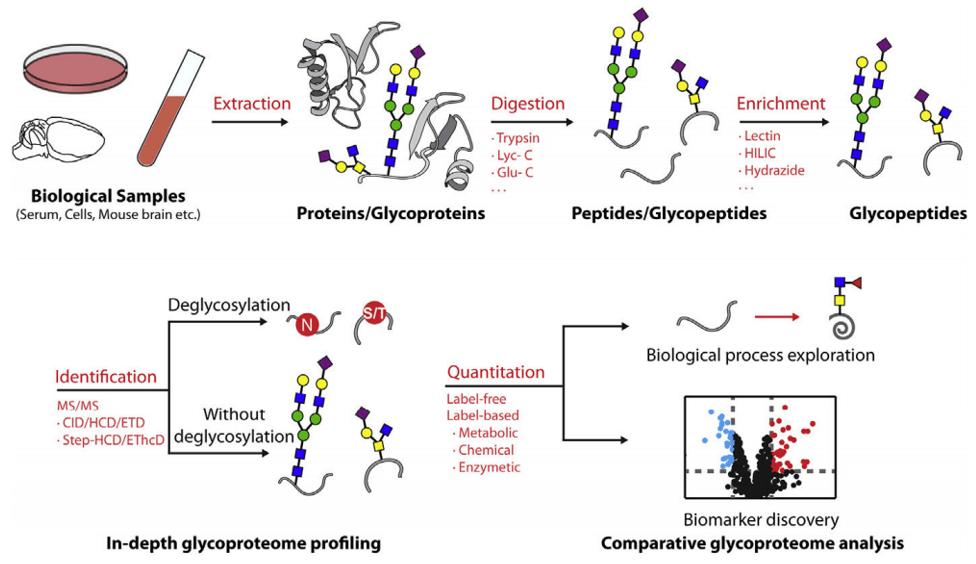 Fig 2. A typical workflow for MS-based glycoproteomics in different complex biological samples.
Fig 2. A typical workflow for MS-based glycoproteomics in different complex biological samples.
Sample requirement
- Serum/plasma: 500 μl/sample.
- Protein: 100 µg
- Anticoagulated blood (EDTA): 1 mL.
- Urine: 1 ml/sample.
- Animal tissues: 200 mg/sample.
- Cells: ≥ 1 × 107/sample.
- Feces: 500 mg/sample
Repeated freezing and thawing of samples must be avoided. The serum sample should be precipitated in the collection tube for 30 minutes at room temperature, then transported to the centrifuge tube and centrifuged at 8000 rpm for 5 minutes. After centrifugation, the supernatant was equally divided into a freezing tube of 500 uL / sample.
Anticoagulants and preservatives must be added immediately after collection and then frozen at -80 °C.
Urine samples should be equally divided into centrifuge tubes with 1 mL per tube, each tube is added with 1/100 (w/v) sodium azide and stored at -80 °C.
Samples should be frozen in liquid nitrogen immediately and then transported to -80 °C for storage after collected.
Cytoactive should be terminated immediately to maintaining cell integrity.
In general, to assure enough sample to fulfill the whole project, the volume of the single sample need to be offered as much as possible. The remaining samples will be stored for one year free of charge and returned at any time if necessary. All samples need to be stored and transported at -80°C and try to avoid using surfactants (SDS, Triton-X) and inorganic salts.
Clinical samples are repeated in no less than 30 cases in a single group.
Animal samples are repeated in no less than 9 cases in a single group.
Report delivery
- Experimental procedure
- Parameters of HPLC and MS
- Raw data, chromatograms and mass spectra
- Metabolites quantification data
- Custom analysis report
Service cycle
- Sample testing: 5-10 working days
- Data analysis: 5-10 working days
Creative Proteomics metabolism analysis platform is committed to the all-around, reliable and accurate analysis service for a variety of target substances, which is suitable for life-science research, drug exploration, biological determination and other fields. We sincerely hope to cooperate with you to assistant your scientific research.
References
- Guzman-Aranguez, P. Argueso. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf, 2010, 8(1):8-17.
- Brockhausen, H. Schachter, P. Stanley, O-GalNAc Glycans, in Essentials of Glycobiology, nd, et al., Editors, Cold Spring Harbor (NY). 2009.
- Z. Chen, J. Huang, L. Li. Recent advances in mass spectrometry (MS)-based glycoproteomics in complex biological samples. Trends Analyt Chem, 2019, 118:880-892.