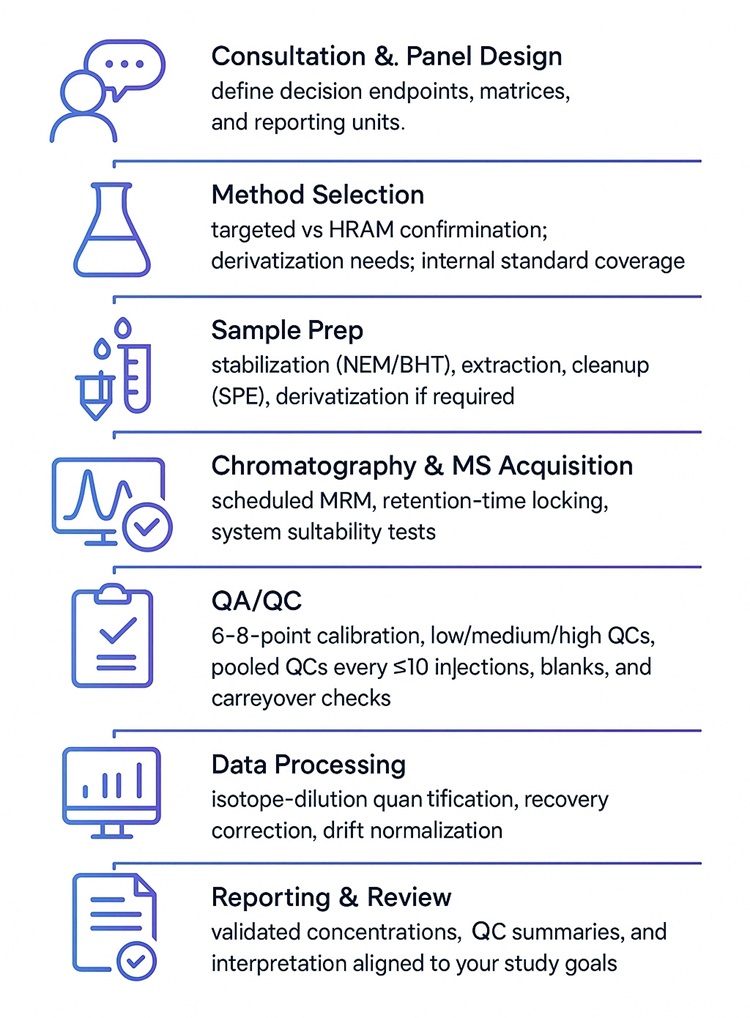
Oxidative Stress Marker Analysis Service
Uncover oxidative stress mechanisms with high-sensitivity LC-MS/MS panels — tailored marker analysis that delivers clarity, accuracy, and confidence.
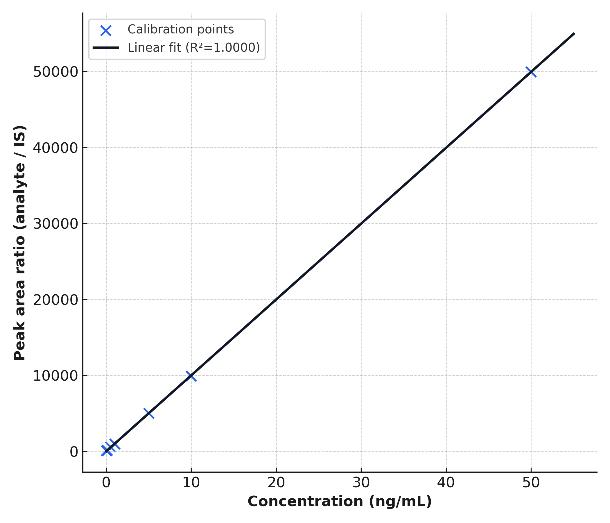
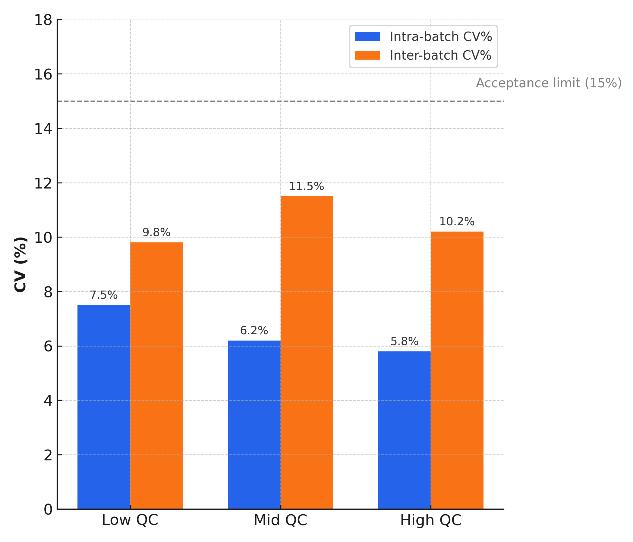
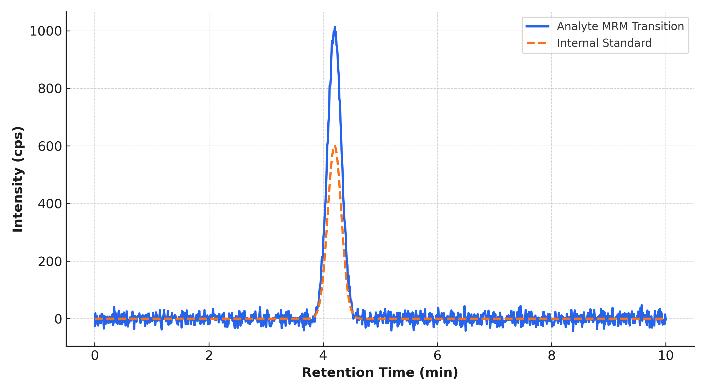
- Why us? Sub-ng/mL sensitivity, isotope-dilution accuracy, QC-driven reproducibility
- What we provide? Targeted multi-marker panels across lipid, protein, DNA, redox, and nitrosative pathways
- What problem we solve? Detect unstable, low-abundance markers with reliable comparability across samples and studies
Explore how our customizable panels and validated workflow can strengthen your research decisions.
CONTACT EXPERT